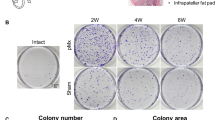
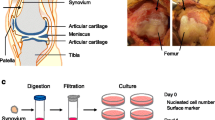
Abstract
Background
Mesenchymal stem cells (MSCs) in synovial fluid increase after traumatic meniscus injuries. However, MSC kinetics in synovial fluid may differ for knees with degenerative meniscus injuries. Furthermore, the combination of surgical repair and synovial MSC transplantation has been found to improve clinical symptoms in patients with degenerative meniscus injury, and in this treatment, only the operation procedure without MSC transplantation might increase MSCs in synovial fluid; if so, soluble factors in synovial fluid will be involved. The purpose is this study was to examine whether MSCs exist in synovial fluid of knees with degenerative meniscus injury, to investigate whether MSCs in synovial fluid increase after harvest of synovium and meniscus repair, and to explore what soluble factors in synovial fluids affect the number of MSCs in synovial fluid.
Methods
Subjects were 7 patients with degenerative meniscus injury who underwent meniscal repair and synovial MSC transplantation. Synovial fluid (Pre) was aspirated from knees before harvest of synovium and meniscus repair. After 2 weeks, synovial fluid (Post) was aspirated again before transplantation of synovial MSCs. A half volume of the synovial fluid was plated and cultured for 2 weeks to count the colony formation. The other half was used for antibody array analysis, and the correlation coefficients between the signal intensity and colony number were measured in 503 factors. Factors with high correlation coefficients were verified by migration assay.
Results
While cell colonies derived from synovial fluid (Pre) were hardly observed, greater numbers of colonies from synovial fluid (Post) were demonstrated. Of the 503 factors, calcitonin gene-related peptide (CGRP) and hepatocyte growth factor (HGF) had high correlation coefficients between colony number and expression level. Both CGRP and HGF promoted migration of synovial fluid MSCs.
Conclusions
MSCs in synovial fluid were hardly seen in knees with degenerated meniscus injury. They significantly increased 2 weeks after harvest of synovium and meniscus repair. Both CGRP and HGF in synovial fluid can possibly induce MSCs from synovium into synovial fluid.

Graphical abstract






Similar content being viewed by others
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- CGRP:
-
calcitonin gene-related peptide
- HGF:
-
hepatocyte growth factor
- OA:
-
osteoarthritis
- α-MEM:
-
α-modified essential medium
- HBSS:
-
Hank’s balanced salt solution
- BMP-2:
-
bone morphogenetic protein 2
References
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317.
Han Y, Li X, Zhang Y, Chang F, Ding J. (2019). Mesenchymal Stem Cells for Regenerative Medicine. Cells. 8(8).
Hou, L., Cao, H., Wang, D., Wei, G., Bai, C., Zhang, Y., & Pei, X. (2003). Induction of umbilical cord blood mesenchymal stem cells into neuron-like cells in vitro. International Journal of Hematology, 78(3), 256–261.
Xu, W., Zhang, X., Qian, H., Zhu, W., Sun, X., Hu, J., Zhou, H., & Chen, Y. (2004). Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Experimental Biology and Medicine (Maywood, N.J.), 229(7), 623–631.
Banas, A., Teratani, T., Yamamoto, Y., Tokuhara, M., Takeshita, F., Quinn, G., Okochi, H., & Ochiya, T. (2007). Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology., 46(1), 219–228.
Katikireddy, K. R., Dana, R., & Jurkunas, U. V. (2014). Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells, 32(3), 717–729.
Cislo-Pakuluk, A., & Marycz, K. (2017). A promising tool in retina regeneration: Current perspectives and challenges when using Mesenchymal progenitor stem cells in veterinary and human ophthalmological applications. Stem Cell Reviews and Reports, 13(5), 598–602.
Sun, Z., Wang, Y., Gong, X., Su, H., & Han, X. (2012). Secretion of rat tracheal epithelial cells induces mesenchymal stem cells to differentiate into epithelial cells. Cell Biology International, 36(2), 169–175.
Qi, Y., Jiang, D., Sindrilaru, A., Stegemann, A., Schatz, S., Treiber, N., Rojewski, M., Schrezenmeier, H., Vander Beken, S., Wlaschek, M., Böhm, M., Seitz, A., Scholz, N., Dürselen, L., Brinckmann, J., Ignatius, A., & Scharffetter-Kochanek, K. (2014). TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. The Journal of Investigative Dermatology, 134(2), 526–537.
Nejadnik, H., Hui, J. H., Feng Choong, E. P., Tai, B. C., & Lee, E. H. (2010). Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. The American Journal of Sports Medicine, 38(6), 1110–1116.
Nicpoń, J., Marycz, K., & Grzesiak, J. (2013). Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Polish Journal of Veterinary Sciences, 16(4), 753–754.
Kondo, S., Nakagawa, Y., Mizuno, M., Katagiri, K., Tsuji, K., Kiuchi, S., Ono, H., Muneta, T., Koga, H., & Sekiya, I. (2019). Transplantation of aggregates of autologous synovial Mesenchymal stem cells for treatment of cartilage defects in the femoral condyle and the femoral groove in Microminipigs. The American Journal of Sports Medicine, 47(10), 2338–2347.
Morito, T., Muneta, T., Hara, K., Ju, Y. J., Mochizuki, T., Makino, H., Umezawa, A., & Sekiya, I. (2008). Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford, England), 47(8), 1137–1143.
Sekiya, I., Ojima, M., Suzuki, S., Yamaga, M., Horie, M., Koga, H., Tsuji, K., Miyaguchi, K., Ogishima, S., Tanaka, H., & Muneta, T. (2012). Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. Journal of Orthopaedic Research, 30(6), 943–949.
Matsukura, Y., Muneta, T., Tsuji, K., Koga, H., & Sekiya, I. (2014). Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clinical Orthopaedics and Related Research, 472(5), 1357–1364.
Horie, M., Sekiya, I., Muneta, T., Ichinose, S., Matsumoto, K., Saito, H., Murakami, T., & Kobayashi, E. (2009). Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells, 27(4), 878–887.
Hatsushika, D., Muneta, T., Nakamura, T., Horie, M., Koga, H., Nakagawa, Y., Tsuji, K., Hishikawa, S., Kobayashi, E., & Sekiya, I. (2014). Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthritis and Cartilage, 22(7), 941–950.
Hatsushika, D., Muneta, T., Horie, M., Koga, H., Tsuji, K., & Sekiya, I. (2013). Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. Journal of Orthopaedic Research, 31(9), 1354–1359.
Kondo, S., Muneta, T., Nakagawa, Y., Koga, H., Watanabe, T., Tsuji, K., Sotome, S., Okawa, A., Kiuchi, S., Ono, H., Mizuno, M., & Sekiya, I. (2017). Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. Journal of Orthopaedic Research, 35(6), 1274–1282.
Nakagawa, Y., Muneta, T., Kondo, S., Mizuno, M., Takakuda, K., Ichinose, S., Tabuchi, T., Koga, H., Tsuji, K., & Sekiya, I. (2015). Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis and Cartilage, 23(6), 1007–1017.
Bin, S. I., Kim, J. M., & Shin, S. J. (2004). Radial tears of the posterior horn of the medial meniscus. Arthroscopy., 20(4), 373–378.
Herrlin, S. V., Wange, P. O., Lapidus, G., Hållander, M., Werner, S., & Weidenhielm, L. (2013). Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee Surgery, Sports Traumatology, Arthroscopy, 21(2), 358–364.
Beaufils, P., Becker, R., Kopf, S., Englund, M., Verdonk, R., Ollivier, M., & Seil, R. (2017). Surgical management of degenerative meniscus lesions: The 2016 ESSKA meniscus consensus. Knee Surgery, Sports Traumatology, Arthroscopy, 25(2), 335–346.
Englund, M., Roemer, F. W., Hayashi, D., Crema, M. D., & Guermazi, A. (2012). Meniscus pathology, osteoarthritis and the treatment controversy. Nature Reviews Rheumatology, 8(7), 412–419.
Kopf S, Beaufils P, Hirschmann MT, Rotigliano N, Ollivier M, Pereira H, et al. (2020). Management of traumatic meniscus tears: the 2019 ESSKA meniscus consensus. Knee Surgery, Sports Traumatology, Arthroscopy
Sekiya, I., Koga, H., Otabe, K., Nakagawa, Y., Katano, H., Ozeki, N., Mizuno, M., Horie, M., Kohno, Y., Katagiri, K., Watanabe, N., & Muneta, T. (2019). Additional use of synovial Mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: A case report. Cell Transplantation, 28(11), 1445–1454.
Baboolal, T. G., Khalil-Khan, A., Theodorides, A. A., Wall, O., Jones, E., & McGonagle, D. (2018). A novel arthroscopic technique for intraoperative mobilization of synovial Mesenchymal stem cells. The American Journal of Sports Medicine, 46(14), 3532–3540.
Brophy, R. H., Sandell, L. J., & Rai, M. F. (2017). Traumatic and degenerative meniscus tears have different gene expression signatures. The American Journal of Sports Medicine, 45(1), 114–120.
Sauerschnig, M., Stolberg-Stolberg, J., Schulze, A., Salzmann, G. M., Perka, C., & Dynybil, C. J. (2014). Diverse expression of selected cytokines and proteinases in synovial fluid obtained from osteoarthritic and healthy human knee joints. European Journal of Medical Research, 19, 65.
Ponte, A. L., Marais, E., Gallay, N., Langonné, A., Delorme, B., Hérault, O., Charbord, P., & Domenech, J. (2007). The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells, 25(7), 1737–1745.
Baek, S. J., Kang, S. K., & Ra, J. C. (2011). In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Experimental & Molecular Medicine, 43(10), 596–603.
Qiu, Y., Marquez-Curtis, L. A., & Janowska-Wieczorek, A. (2012). Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy., 14(3), 285–295.
Kohno, Y., Mizuno, M., Ozeki, N., Katano, H., Otabe, K., Koga, H., Matsumoto, M., Kaneko, H., Takazawa, Y., & Sekiya, I. (2018). Comparison of mesenchymal stem cells obtained by suspended culture of synovium from patients with rheumatoid arthritis and osteoarthritis. BMC Musculoskeletal Disorders, 19(1), 78.
Mills, P. M., Wang, Y., Cicuttini, F. M., Stoffel, K., Stachowiak, G. W., Podsiadlo, P., & Lloyd, D. G. (2008). Tibio-femoral cartilage defects 3-5 years following arthroscopic partial medial meniscectomy. Osteoarthritis and Cartilage, 16(12), 1526–1531.
Vu, T. H., & Werb, Z. (2000). Matrix metalloproteinases: Effectors of development and normal physiology. Genes & Development, 14(17), 2123–2133.
Beaufils, P., & Pujol, N. (2017). Management of traumatic meniscal tear and degenerative meniscal lesions. Save the meniscus. Orthopaedics & Traumatology, Surgery & Research, 103(8S), S237–SS44.
Englund, M., Guermazi, A., Roemer, F. W., Aliabadi, P., Yang, M., Lewis, C. E., Torner, J., Nevitt, M. C., Sack, B., & Felson, D. T. (2009). Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The multicenter osteoarthritis study. Arthritis and Rheumatism, 60(3), 831–839.
Mochizuki, T., Muneta, T., Sakaguchi, Y., Nimura, A., Yokoyama, A., Koga, H., & Sekiya, I. (2006). Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis and Rheumatism, 54(3), 843–853.
Kilo, S., Harding-Rose, C., Hargreaves, K. M., & Flores, C. M. (1997). Peripheral CGRP release as a marker for neurogenic inflammation: A model system for the study of neuropeptide secretion in rat paw skin. Pain., 73(2), 201–207.
Onuma, H., Tsuji, K., Hoshino, T., Inomata, K., Udo, M., Nakagawa, Y., Katagiri, H., Miyatake, K., Watanabe, T., Sekiya, I., Muneta, T., & Koga, H. (2020). Fibrotic changes in the infrapatellar fat pad induce new vessel formation and sensory nerve fiber endings that associate prolonged pain. Journal of Orthopaedic Research, 38, 1296–1306.
Larsson, J., Ekblom, A., Henriksson, K., Lundeberg, T., & Theodorsson, E. (1989). Immunoreactive tachykinins, calcitonin gene-related peptide and neuropeptide Y in human synovial fluid from inflamed knee joints. Neuroscience Letters, 100(1–3), 326–330.
Larsson, J., Ekblom, A., Henriksson, K., Lundeberg, T., & Theodorsson, E. (1991). Concentration of substance P, neurokinin a, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid from knee joints in patients suffering from rheumatoid arthritis. Scandinavian Journal of Rheumatology, 20(5), 326–335.
Zhang, Y., Yang, J., Zhang, P., Liu, T., Xu, J., Fan, Z., Shen, Y., Li, W., & Zhang, H. (2016). Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Scientific Reports, 6, 27724.
Chen, J., Ma, G., Liu, W., Liu, Y., & Ding, Y. (2017). The influence of the sensory neurotransmitter calcitonin gene-related peptide on bone marrow mesenchymal stem cells from ovariectomized rats. Journal of Bone and Mineral Metabolism, 35(5), 473–484.
Fukushima T, Uchiyama S, Tanaka H, Kataoka H(2018) Hepatocyte Growth Factor Activator: A Proteinase Linking Tissue Injury with Repair. International Journal Molecular Science 19(11).
Mabey, T., Honsawek, S., Saetan, N., Poovorawan, Y., Tanavalee, A., & Yuktanandana, P. (2014). Angiogenic cytokine expression profiles in plasma and synovial fluid of primary knee osteoarthritis. International Orthopaedics, 38(9), 1885–1892.
Cottrell, G. S. (2019). CGRP receptor Signalling pathways. Handbook of Experimental Pharmacology, 255, 37–64.
Ilangumaran, S., Villalobos-Hernandez, A., Bobbala, D., & Ramanathan, S. (2016). The hepatocyte growth factor (HGF)-MET receptor tyrosine kinase signaling pathway: Diverse roles in modulating immune cell functions. Cytokine., 82, 125–139.
Marycz, K., Mierzejewska, K., Śmieszek, A., Suszynska, E., Malicka, I., Kucia, M., et al. (2016). Endurance exercise mobilizes developmentally early stem cells into peripheral blood and increases their number in bone marrow: Implications for tissue regeneration. Stem Cells International, 2016, 5756901.
Marycz, K., Kornicka, K., Grzesiak, J., Śmieszek, A., & Szłapka, J. (2016). Macroautophagy and selective Mitophagy ameliorate Chondrogenic differentiation potential in adipose stem cells of equine metabolic syndrome: New findings in the field of progenitor cells differentiation. Oxidative Medicine and Cellular Longevity, 2016, 3718468.
Acknowledgments
We thank Ms. Mika Watanabe and Ms. Kimiko Takanashi for the management of our laboratory and Tina Hilling for English editing.
Availability of Data and Materials
Not applicable.
Funding
This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant JP19bk0104065.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or critically revising it for important intellectual content, and all authors approved the final manuscript. I.S. had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. N.W., K.E., and I.S. designed and performed the research, analyzed and interpreted the data, and drafted the manuscript. N.W. performed the experiments and collected the data. K.K. made substantial contributions to multidifferentiation analysis, flowcytometry, and protein array analysis. N.O., M.M., K.O., H.Ka., and Y.K. made substantial contributions to the analysis and interpretation of the data. K.T. made substantial contributions to the study design for migration assay. H.Ko. performed arthroscopy and collected synovial fluid from the patients.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
This study was approved by the Medical Research Ethics Committee of Tokyo Medical and Dental University, and informed consent was obtained from all study subjects.
Consent for Publication
All indivisuals consented to publication.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Watanabe, N., Endo, K., Komori, K. et al. Mesenchymal Stem Cells in Synovial Fluid Increase in Knees with Degenerative Meniscus Injury after Arthroscopic Procedures through the Endogenous Effects of CGRP and HGF. Stem Cell Rev and Rep 16, 1305–1315 (2020). https://doi.org/10.1007/s12015-020-10047-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10047-0