Abstract
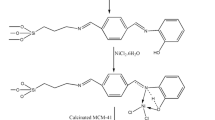
Synthesis of efficient and reusable catalyst was carried out by immobilization of azacrown ether onto the covalently linked amine-functionalized mesoporous MCM-41 via a simple method. Application of this linked azacrown ether functionalized MCM-41 catalyst was also investigated as an effective, heterogeneous and host material in the regioselective ring opening and triazole cyclization of epoxides by phenylacetylene and sodiumazide in click reaction. The heterogeneous catalyst was characterized by TGA,XRD,TEM, 1HNMR, 13CNMR and FT-IR techniques. The results of theses analysis showed that, MCM-41@azacrown-Cu with a 22.99% mol loading of copper has given excellent catalytic activity and high efficiency in short time. It is noted that one of the most valuable factors in this work is the recyclability of the catalyst.










Similar content being viewed by others
References
T.V. Kovalchuk, H. Sfihi, A.S. Korchev, A.S. Kovalenko, V.G. Il'in, V.N. Zaitsev, J. Phys. Chem. B 109 (2005)
V. Jarian, M. Vera, C. Pegie, Beilstein. J. Nanotechnol. 2, (2011) Doi:10.3762/bjnano.2.87
C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, J. Nat 359, 710–712 (1992)
M. Kato, T. Shigeno, T. Kimura, K. Kuroda, Chem. Mater 17, 25 (2005). https://doi.org/10.1021/cm0505749
D. Xueya, S. Hua, S. Hualin, G. Jing, L. Feng, L. Yanxiu, React. Kinet. Catal. Lett 44, 4 (2019). https://doi.org/10.1177/1468678319825909
R. Narayan, U.Y. Nayak, A.M. Raichur, S. Garg, Advances. Pharmaceutics 10, 3 (2018). https://doi.org/10.3390/pharmaceutics10030118
H. Sharghi, R. Khalifeh, A. R. Salimi Beni, I. R .J. Iran. Chem. Soc 7, 275–288 (2010).
K. Maeda, N. Shinyashiki, S. Yagihara, S. Wiegand, R. Kita, J. Chem. Phys 143, 124504 (2015). https://doi.org/10.1063/1.4931115
S. Barmana, M. Nath Roy, RSC. Adv 6, 76381–76389 (2016). Doi:10.1039/C6RA14138B
G. Chehardoli, A. Bahmani, Supramol. Chem. 31(4), 221 (2019). https://doi.org/10.1080/10610278.2019.1568432
C. Yoo, H. M. Dodgea, A. J. M. Miller, Chem. Comm 00 (2019). Doi:10.1039/C9CC00803A
T. Kojima, F. Ogishima, T. Nishibu, H. Kotani, T. Ishizuka, T. Okajima, S. Nozawa, Y. Shiota, K. Yoshizawa, H. Ohtsu, M. Kawano, T. Shiga, H. Oshio, Inorg. Chem 57, 9683–9695 (2018). https://doi.org/10.1021/acs.inorgchem.8b00037
A. Viso, R. Fernández de la Pradilla, A. García, A. Flores, Chem. Rev 105 (2005). Doi:10.1021/cr0406561
S. H. Sumrra, M. Ibrahim, S. Ambreen, M. Imran, M. Danish, F. Sultana Rehmani, Bioinorg. Chem. Appl (2014). Doi:10.1155/2014/812924
M. Whiting, J. Muldoon, Y.C. Lin, S.M. Silverman, W. Lindstrom, A.J. Olson, H.C. Kolb, M.G. Finn, K.B. Sharpless, Elder JH, Fokin VV. Angew. Chem. Int. Ed 45, 9 (2006). https://doi.org/10.1002/anie.200502161
Y. Bourne, H.C. Kolb, Z. Radić, K.B. Sharpless, P. Taylor, P. Marchot, Natl. Acad. Sci. USA 101, 6 (2004). https://doi.org/10.1073/pnas.0308206100
J. Thomas, J. John, N. Parekh, W. Dehaen, Angew. Chem. Int. Ed. Engl 53, 38 (2014). https://doi.org/10.1002/anie.201403453
T. W. Kim, Y. Yong, S. Y. Shin, H. Jung, K. H. Park, Y. H. Lee, Y. Lim, K. Y. Jung. Bioorg. Chem 59 (2015). Doi:10.1016/j.bioorg
A. Faruk, K.D. Biplob, J. Pharm. Chem. Biol. Sci 2(4), 235–247 (2014)
W. G. Lewis, L. G. Green, F. Grynszpan, Z. Radic, P. R. Carlier, P. Taylor, M. G. Finn, K. B. Sharpless, Angew. Chem 114 (2002). Doi:10.1002/1521–3757(20020315)114:6
N. Akira, K. Hisashi, S. Taketoshi, S. Hirosada, K. Morio, S. Yasushi. J. Med. Chem 20 (1977). Doi:10.1021/jm00211a030
G. Ye, T. Lan, Z.X. Huang, X.N. Cheng, C.Y. Cai, S.M. Ding, M.L. Xie, Eur. J. Med. Chem 1, 362–373 (2019). https://doi.org/10.1016/j.ejmech.2019.05.045
T. El Malah, H. F. Nour, A. Satti, B. A. Hemdan, W. A. El-Sayed Molecules 25, 4 790 (2020). Doi:10.3390/molecules25040790
A. R. Modarresi Alam, M. Nasrollahzadeh, Tetrahedron. Lett 33 (2009). Doi:10.3906/kim-0808–44
V. A. Ostrovskii, R. E. Trifonov, E. A. Popova, Russ. Chem. Bull 61 (2012).
C. Menendez, A. Chollet, F. Rodriguez, C. Inard, M. R. Pasca, C. Lherbet, M. Baltas, Eur. J. Med. Chem 52 (2012). Doi:10.1016/j.ejmech
E.S. Hadi, E. Hossien, L. Jalil, R. Amin, Appl. Organomet. Chem 32, 1 (2017). https://doi.org/10.1002/aoc.3947
V. A. Blanca Ivonne, L. R. Leticia, A. B. Deyanira, N. S. Guillermo Enrique, G. C. Atilano, H. L. Víctor, M. S. José Antonio, J. Tetrahedron. Lett 58 (2017). Doi:10.1016/j.tetlet.2017.05.055
J. L. Li, Y. Q. Zhang, Y. Zhang, A. L. Zhu, J. S. Zhang, Chinese. Chemical. Letters 25 (2010). Doi:10.1016/j.cclet.2014.03.004
M.C. Giel, C.J. Smedley, E.R.R. Mackie, T. Guo, J. Dong, T.P.S. da Costa, J. E. Moses Chem. Rxiv 211, 2464 (2019). https://doi.org/10.26434/chemrxiv.9937754.v1
D.H. Christopher, L. Xin-Ming, W. Dong, Pharm. Res 25, 10 (2008). https://doi.org/10.1007/s11095-008-9616-1
V. R. Vsevolod, G. G. Luke, V. F. Valery, S. K Barry, Angew. Chem 41 (2002). Doi:10.1002/1521–3773(20020715)41
A. Heather, D.R. Rosemary, W.M. Steven, N. Arundhati, Y. Woon-Seok, E.H. Jason, M.P. Suresh, A.T. Abdul, M.B. Vanessa, J.K. Russell, V.F. Valery, S. Barry, R.H. James, Angew Chem Int Ed Engl 48, 27 (2009). https://doi.org/10.1002/anie.200900488
D.B. Jovica, B. Vincenzo, C. Alberto, N.L. James, S. Serena, S. Fraser, Chem. Eur. J 10, 8 (2002). https://doi.org/10.1002/chem.200305687
S. Díez González, A. Correa, L. Cavallo, S. P. Nolan, Chem. Eur .J 12 (2006).
O.S. Miljanić, W.R. Dichtel, S.I. Khan, S. Mortezaei, J.R. Heath, J.F. Stoddart, J. Am. Chem. Soc 129, 26 (2007)
F. Grégory, K. Ashok, Chem. Commun 0 (2008). Doi:10.1039/B809870K
S. Li, L. Wang, F. Yu, Z. Zhu, D. Shobaki, H. Chen, M. Wang, J. Wang, G. Qin, U.J. Erasquin, L. Ren, Y. Wangb, C. Cai, Chem. Soi 8(3), 2107–2114 (2017)
W.R. Dichtel, O.S. Miljanić, J.M. Spruell, J.R. Heath, J.F. Stoddart, J. Am. Chem. Soc 128, 32 (2006). https://doi.org/10.1021/ja063127i
M. Michael, S. Kristin, D. Eric, J.H. Craig, P.R. Thomas, W. Peng, V.F. Valery, Macromolecules 38(9), 3663 (2005). https://doi.org/10.1021/ma047657f
L. Yu Heng, J. R. Peter, W. Michael, H. T. Matthew, Chem. Soc. Rev 40 (2011). Doi:10.1039/C0CS00143K
G. A. Burley, J. Gierlich, M. R. Mofid, H. Nir, S. Tal, Y. Eichen, T. Carell, J. Am. Chem. Soc 128 (2006). Doi:10.1021/ja055517v
T. Ishizuka, H.S. Liu, K. Ito, Y. Xu, Sci. Rep 6, 33217 (2016). https://doi.org/10.1038/srep33217
R. Mohammad Reza, G. Shahriar, T. Mahbobeh, S. Sajjad J Med Chem 13, 1 (2013). Doi:10.5829/idos
Acknowledgements
We gratefully acknowledge the support of this work by Yasouj University Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
shirali, S., Beni, A.S. Preparation and characterization of novel hybrid nanomaterial catalyst MCM-41@AzaCrown-SB-Cu and its application in synthesis of 1, 2, 3-triazole derivatives in click chemistry. J Porous Mater 27, 1601–1611 (2020). https://doi.org/10.1007/s10934-020-00924-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-020-00924-x