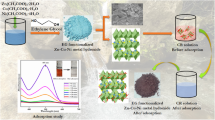
Abstract
The present work seeks to investigate the kinetics and thermodynamic studies of ethidium bromide (EtBr) and eosin adsorption onto the synthesized Manganese (II) doped Zinc (II) Sulphide nanoparticles. A convenient scheme of co-precipitation was used for the synthesis of Manganese (II) doped Zinc (II) Sulphide nanoparticles. The Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (FESEM) and X-ray diffractogram (XRD) techniques were used for the characterization of synthesized nanoparticles. The adsorption study was undertaken in a systematic manner. Effects of different experimental parameters were studied using batch adsorption method. It was evident from the results that EtBr and eosin removal was inversely proportional to the concentration of initial dye and directly proportional to contact time and adsorbent used. To study the adsorption equilibrium three different isotherm models like Langmuir, Freundlich and Flory-Huggins were used. It was observed that adsorption data synced most successfully with Langmuir isotherm model as compared to Freundlich and Flory-Huggins isotherm model. To fit the investigational statistics, the kinetic models pseudo 1st order, pseudo 2nd order and intra particle diffusion were taken onto consideration. The maximum dye removal of 98.19% and 97.16% for EtBr and eosin, was observed during the synthesis of nanoparticles.













Similar content being viewed by others
References
Ahamad T, Ruksana, Chaudhary AA, Naushad M, Alshehri SM, (2019) Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. International Journal of Biological Macromolecules 134:180–188
Akbari H, Gholami M, Akbari H, Adibzadeh A, Taghavi L, Hayati B, et al. Poly (amidoamine) generation 6 functionalized Fe 3O4@SiO2/GPTMS core–shell magnetic NPs as a new adsorbent for Arsenite adsorption: kinetic, isotherm and thermodynamic studies. J Environ Health Sci Eng. 2020;18:23–6.
Cao J, Yang J, Zhang Y, Yang L, Wang Y, Wei M, et al. Optimized doping concentration of manganese in zinc sulphide nanoparticles for yellow orange light emission. J Alloy Compd. 2009;486:890–4.
Chattopadhyay M, Kumbhakar P, Tiwary CS, Sarkar R, Mitra K, Chatterjee U. Multiphoton absorption and reflection in Mn+2 doped ZnS quantum dots. J Appl Phys. 2009;105:024313.
Chavoshan S, Khodadadi M, Nasseh N. Photocatalytic degradation of penicillin G from simulated wastewater using the UV/ZnO process: isotherm and kinetic study. J Environ Health Sci Eng. 2020;18:107–17.
Devi BSR, Raveendran R, Vaidyan AV. Synthesis and characterization of Mn+2 doped ZnS nanoparticles. Pramana. 2007;68(4):679–87.
Dogan M, Abak H, Alkan M. Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. J.Hazard.Mater. 2009a;164:172–81.
Dogan M, Karaoglu MH, Alkan M. Adsorption kinetics of maxilon yellow 4GL and maxilon red GRL dyes on kaolinite. J.Hazard.Mater. 2009b;165:1142–51.
Dong B, Cao L, Su J, Liu W, Qu H, Zhai H. Water soluble ZnS:Mn/ZnS core/shell nanoparticles prepared by a novel two-step method. J Alloy Compd. 2010;492:363–7.
Elwakeel KZ, Elgarahy AM, Elshoubaky GA, Mohammad SH. Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. J Environ Health Sci Eng. 2020;18:35–50.
Oyelude EO, Awudza JAM, Twumasi SK. Equilibrium, kinetic and thermodynamic study of removal of eosin yellow from aqueous solution using teak leaf litter powder. Sci Rep. 2017;7:1–10.
Fang X, Bando Y, Gautam UK, Zhai T, Zeng H, Xu X, et al. ZnO and ZnS nanostructures: ultraviolet-light emitters, lasers and sensors. Crit Rev Solid State. 2004;34:190–223.
Hu H, Zhang W. Synthesis and properties of transition metals and rare-earth metals doped ZnS nanoparticles. Opt Mater. 2006;28:536–50.
Naushad M, Mittal A, Rathore M, Gupta V. Ion-exchange kinetic studies for Cd(II), Co(II), Cu(II), and Pb(II) metal ions over a composite cation exchanger. Desalin. Water Treat. 2015;54:2883–90
Kahrizi P, Fatemeh S, Mohseni-Shahri MF. Adsorptive removal of cadmium from aqueous solutions using NiFe2O4/hydroxyapatite/graphene quantum dots as a novel nano-adsorbent. J Nanostruct Chem. 2018;8(4):441–52.
Kaith BS, Saruchi, Jindal R, Bhatti MS. Screening and RSM optimization for synthesis of a gum tragacanth–acrylic acid based device for in situ controlled cetirizine dihydrochloride release. Soft Matter. 2012;8:2286–93.
Kaur B, Chand S, Singh K, Malik AK. Detoxification of dye contaminated water by Mn+2 – doped ZnS nanostructures. Bull Mater Sci. 2019;42:61–70.
Kaur N, Kaur S, Singh J, Rawat M. A review on zinc sulphide nanoparticles: from synthesis, properties to applications. Journal of Bioelectronics and nanotechnology. 2016;1(1):1–5.
Khoshsang H, Ghaffarinejad A, Kazemi H, Wang Y. Hamidreza Arandiyan, one-pot synthesis of S-doped Fe2O3/C magnetic nanocomposite as an adsorbent for anionic dye removal: equilibrium and kinetic studies. J Nanostruct Chem. 2018;1(8):23–32.
Kole AK, Kumbhakar P. Effect of manganese doping on the photoluminescence characteristics of chemically synthesized zinc sulphide nanoparticles. Appl Nanosci. 2012;2(1):15–23.
Kumar V, Rehani V, Kaith BS, Saruchi. Synthesis of a biodegradable interpenetrating polymer network of Av-cl-poly(AA-ipn-AAm) for malachite green dye removal: kinetics and thermodynamic studies. RSC Adv. 2018;8:41920–38.
Li, X., Li, Y.. Adsorptive Removal of Dyes from Aqueous Solution by KMnO4-Modified Rice Husk and Rice Straw. 2019, Article ID 8359491, 9. https://doi.org/10.1155/2019/8359491, 9.
Liu J, Wang N, Zhang H, Baeyens J. Adsorption of Congo red dye on FexCo3-xO4 nanoparticles. J Environ Manag. 2019a;238:473–83.
Liu QX, Zhou YR, Wang M, Zhang Q, Chen TY, Yu DC. Adsorption of methylene blue from aqueous solution onto viscose-based activated carbon fiber felts: kinetics and equilibrium studies. Adsorpt Sci Technol. 2019b;37(3–4):312–32.
Lu C, Chiu H, Liu C. Removal of zinc(II) from aqueous solution by purified carbon nanotubes: kinetics and equilibrium studies. Ind Eng Chem Res. 2006;45:2850–5.
Mittal H, Mishra SB. Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohydrate Polymer. 2014;101:1255–64.
Mittal H, Kumar V, Saruchi RSS. Adsorption of methyl violet from aqueous solution using gum xanthan/Fe3O4 based nanocomposite hydrogel. Int J Biol Macromol. 2016;89:1–11.
Ahmed DN, Naji LA, Faisal AAH, Al-Ansari N, Naushad M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Scientific Reports. 2020;10 (1): 1–12.
Moradi O, Norouzi M, Fakhri A, Naddafi K. Interaction of removal Ethidium bromide with carbon nanotube: equilibrium and isotherm studies. J. Environ. Health Sci. Eng. 2014;2014:12:17.
Moradi O, Zare K, Monajjemi M, Yari M, Aghaie H. The studies of equilibrium and thermodynamic adsorption of Pb(II), cd(II) and cu(II) ions from aqueous solution onto SWCNTs and SWCNT -COOH surfaces. Fullerenes, Nanotubes and Carbon Nanostructures. 2010;18:285–302.
Murugadoss G, Ramasamy V. Synthesis, effect of capping agents and optical properties of manganese-doped zinc sulphide nanoparticles. Luminescence. 2013;28(1):69–75.
Yari M, Rajabi M, Moradi O, Yari A, Asif M, Agarwal S, et al. Kinetics of the adsorption of Pb(II) ions from aqueous solutions by graphene oxide and thiol functionalized graphene oxide. Journal of Molecular Liquids. 2015;209:50–7.
Moradi O, Zare K. Adsorption of Pb(II), cd(II) and cu(II) ions from aqueous solution onto SWCNTs and SWCNT -COOH surfaces: kinetics study. Fullerenes, nanotubes and carbon nanostructures. 2011;19:628–52.
Najafi F, Norouzi M, Zare K, Fakhri A. Removal of ethidium bromide b carbon nanotube in aqueous solution: isotherms, equilibrium mechanism studies, and its comparison with nanoscale zero valent iron as adsorbent. Journal of Nanostructure in Chemistry. 2013a;60(3):1–7.
Najafi F, Norouzi M, Zare K, Fakhri A. Removal of ethidium bromide by carbon nanotube in aqueous solution: isotherms, equilibrium mechanism studies, and its comparison with nanoscale of zero valent iron as adsorbent. Journal of Nanostructure in Chemistry. 2013b;3:60.
Noradoun CE, Cheng IF. EDTA degradation induced by oxygen activation in a Zerovalent Iron/air/water system. Environ Part Sci Technol. 2005;39:7158–63.
Noubactep C. Characterizing the discoloration of methylene blue in FeO/H2O systems. J Hazard Mater. 2009;166:79–87.
Okey-Onyesolu CF, Okoye CC, Chime DC. Removal of eosin yellow dye from aqueous solution using oil bean seed shells based activated carbons: equilibrium. Kinetics and thermodynamics studies International Journal of Scientific & Engineering Research. 2018;9(3):140.
Omid M, Ali F, Saeideh A, Sepideh A. Isotherm, thermodynamic, kinetics, and adsorption mechanism studies of Ethidium bromide by single-walled carbon nanotube and carboxylate group functionalized single-walled carbon nanotube. J Colloid Interface Sci. 2013;395:224–9.
Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Del Rev. 2003;55:329–47.
Rajabi M, Mahanpoor K, Moradi O. Thermodynamic and kinetic studies of crystal violet dye adsorption with poly(methyl methacrylate)-graphene oxide and poly(methyl methacrylate)-graphene oxide-zinc oxide nanocomposites. J Appl Polym Sci. 2019a;136(22):47495.
Rajabi M, Mahanpoor K, Moradi O. Preparation of PMMA/GO and PMMA/GO-Fe3O4 nanocomposites for malachite green dye adsorption: kinetic and thermodynamic studies. Compos Part B. 2019b;167:544–55.
Saruchi, Kumar V. Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pb+2 ions from aqueous solutions by a hybrid ion-exchanger. Arab J Chem. 2019a;12:316–29.
Saruchi, Kumar V, Kaith BS. Synthesis of hybrid ion exchanger for rhodamine B dye removal: equilibrium, kinetic and thermodynamic studies. I & EC Research. 2016;55(39):10492–9.
Saruchi, Kaith BS, Kumar V, Jindal R. Biodegradation study of enzymatically catalyzed interpenetrating polymer network: evaluation of agrochemical release and impact on soil fertility. Biotechnol. Rep. 2016;9:74–81.
Saruchi, Kumar, Mittal H, Alhassan SM. Biodegradable hydrogels of tragacanth gum polysaccharide to improve water retention capacity of soil and environment-friendly controlled release of agrochemicals. International J Biol Macromol. 2019;132:1252–61.
Saruchi, Thakur P, Kumar, V. Kinetics and thermodynamic studies for removal of methylene blue dye by biosynthesize copper oxide nanoparticles and its antibacterial activity, Journal of Environmental Health Science and Engineering. 2019b;17:367–76.
Sousa DM, Alves LC, Marques A, Gaspar G, Lima JC, Ferreira T. Facile microwave-assisted synthesis manganese doped zinc sulfide nanoparticles. Sci Rep. 2018;8:15992–6002.
Srivastava R.K., Pandey N., Mishra S., 2013. Effect of cu concentration on the photoconductivity properties of ZnS nanoparticles synthesized by co-precipitation method. Material science in semiconductor processing, 16: 1659-1664.
Naushad M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium, Chem. Eng. J. 2014; 235: 100–8.
Ugbe FA, Ikudayisi VA. The kinetics of eosin yellow removal from aqueous solution using pineapple peels. Edorium J Waste Manag. 2017;2:5–11.
Venkatesha TG, Viswanatha R, Arthoba NY, Chethana BK. Kinetics and thermodynamics of reactive and vat dyes adsorption on MgO nanoparticles. Chem EngJ. 2012;198:1–10.
Vergis BR, Krishna RH, Kottam N, Nagabhushana BM, Sharath R, Darukaprasad B. Removal of malachite green from aqueous solution by magnetic CuFe2O4 nano-adsorbent synthesized by one pot solution combustion method. J Nanostruct Chem. 2018;8(1):1–12.
Koli PB, Kapadnis KH, Deshpande UG, Patil MR. Fabrication and characterization of pure and modified Co3O4 nanocatalyst and their application for photocatalytic degradation of eosine blue dye: a comparative study. J Nanostruct Chem. 2018;4(8):453–63.
Wang H, Zhou A, Peng F, Yu H, Yang J. Mechanism study on adsorption of acidified multi-walled carbon nanotubes to Pb(II). J Colloid Interface Sci. 2007;316:277–83.
Yao Y., Xu F., Chen M., Xu Z., Zhu Z.. Nano/micro engineered and molecular systems (NEMS), in: 5th IEEE international conference, 2010a 1083–1087.
Yao Y, Xu F, Chen V, Xu Z, Zhu Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour Technol. 2010b;101:3040–6.
Zhao, Y., Sun, Q., Zhang, X., Baeyens, J., Su, H., Self-assembled selenium nanoparticles and their application in the rapid diagnostic detection of small cell lung cancer biomarker. Soft Matter. 2017, 14(5) https://doi.org/10.1039/C7SM01687E
Zhou M, Yang T, Hu W, He X, Kie J, Wang P, et al. Scalable fabrication of Metallopolymeric superstructures for highly efficient removal of methylene blue. Nanomaterials. 2019b;9:1–18.
Zhou Y, Lu J, Zhou Y, Liu Y. Recent advances for dyes removal using novel adsorbents: a review. Environ Pollut. 2019a;252A:32–365.
Acknowledgements
Dr. Saruchi and Dr. Vaneet Kumar are thankful to CT Group of institutions, Jalandhar for carring out this research work. This work was also funded by researchers supporting project and one of the authors Asma A. ALOThman is grateful to the researchers supporting project number (RSP-2020/243), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest regarding this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saruchi, Verma, R., Kumar, V. et al. Comparison between removal of Ethidium bromide and eosin by synthesized manganese (II) doped zinc (II) sulphide nanoparticles: kinetic, isotherms and thermodynamic studies. J Environ Health Sci Engineer 18, 1175–1187 (2020). https://doi.org/10.1007/s40201-020-00536-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00536-2