Abstract
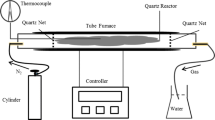
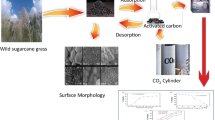
In this work, activated carbon was synthesized from lawn grass via its physical treatment. The lawn grass was washed with hydrochloric acid solution before heat treatment step at different temperatures in the presence of carbon dioxide (CO2) gas. Activated carbon samples obtained from heat treatment process and raw grass samples were completely analyzed using scanning electron microscopy (SEM), thermogravimetric analyzer (TGA), Fourier transforms infrared (FTIR) spectroscopic, and X-ray diffraction (XRD). The surface area of activated carbon resulted from heat treatment of lawn grass in the presence of CO2 was higher, i.e., 208 m2/g, as compared to raw grass, i.e., 0.0068 m2/g. The adsorption capacity was highest (i.e. 0.12 mmol/g at 25 °C and 1 bar for CO2 adsorption) for activated carbon sample prepared at 750 °C. Therefore, activated carbon prepared from heat treatment of lawn grass can be a viable option for pollutants removal by using this low cost and effective adsorbent.







Similar content being viewed by others
Data availability
Not applicable.
References
Rethinasabapathy M, Han J, Chul K, Kang S (2020) Silver grass-derived activated carbon with coexisting micro-, meso- and macropores as excellent bioanodes for microbial colonization and power generation in sustainable microbial fuel cells. Bioresour Technol 300:122646. https://doi.org/10.1016/j.biortech.2019.122646
Charnkeitkong P, Phoophuangpairoj R (2020) Modification of Manila grass activated carbon for reactive dye adsorption from textile printing wastewater modification of Manila grass activated carbon for reactive dye adsorption from textile printing wastewater. In: IOP Conference Series: Materials Science and Engineering. pp 1–8
Mesfer MK Al (2020) Synthesis and characterization of high-performance activated carbon from walnut shell biomass for CO2 capture. Environ Sci Pollut Res 1–9. https://doi.org/10.1007/s11356-020-07934-x RESEARCH ARTICLE Synthesis
Tu B, Wen R, Wang K, Cheng Y, Deng Y, Cao W, Zhang K, Tao H (2020) Efficient removal of aqueous hexavalent chromium by activated carbon derived from Bermuda grass. J Colloid Interface Sci 560:649–658. https://doi.org/10.1016/j.jcis.2019.10.103
Kalyani P, Anitha A, Darchen A (2013) Activated carbon from grass - a green alternative catalyst support for water electrolysis. Int J Hydrog Energy 38:10364–10372. https://doi.org/10.1016/j.ijhydene.2013.06.022
Xu J, Chen L, Qu H, Jiao Y, Xie J, Xing G (2014) Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl Surf Sci 320:674–680. https://doi.org/10.1016/j.apsusc.2014.08.178
Saka C (2012) BET, TG-DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. J Anal Appl Pyrolysis 95:21–24. https://doi.org/10.1016/j.jaap.2011.12.020
Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A (2011) Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Res 45:4047–4055. https://doi.org/10.1016/j.watres.2011.05.016
Kilic M, Apaydin-Varol E, Pütün AE (2011) Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: equilibrium, kinetics and thermodynamics. J Hazard Mater 189:397–403. https://doi.org/10.1016/j.jhazmat.2011.02.051
Hoseinzadeh Hesas R, Wan Daud WMA, Sahu JN, Arami-Niya A (2013) The effects of a microwave heating method on the production of activated carbon from agricultural waste: a review. J Anal Appl Pyrolysis 100:1–11. https://doi.org/10.1016/j.jaap.2012.12.019
Bedin KC, Martins AC, Cazetta AL, Pezoti O, Almeida VC (2016) KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal. Chem Eng J 286:476–484. https://doi.org/10.1016/j.cej.2015.10.099
Antoniou N, Stavropoulos G, Zabaniotou A (2014) Activation of end of life tyres pyrolytic char for enhancing viability of pyrolysis - critical review, analysis and recommendations for a hybrid dual system. Renew Sust Energ Rev 39:1053–1073. https://doi.org/10.1016/j.rser.2014.07.143
Ali R, Aslam Z, Shawabkeh RA et al (2020) BET, FTIR and RAMAN characterizations of activated carbon from waste oil fly ash. Turkish J Chem 44:279–295. https://doi.org/10.3906/kim-1909-20
Bouchelta C, Salah M, Bertrand O (2008) Bellat J. Preparation and characterization of activated carbon from date stones by physical activation with steam 82:70–77. https://doi.org/10.1016/j.jaap.2007.12.009
Nabais JMV, Laginhas C, Carrott MMLR, Carrott PJM, Amorós JEC, Gisbert AVN (2013) Surface and porous characterisation of activated carbons made from a novel biomass precursor, the esparto grass. Appl Surf Sci 265:919–924. https://doi.org/10.1016/j.apsusc.2012.11.164
Aslam Z, Hussein IA, Shawabkeh RA, Parvez MA, Ahmad W, Ihsanullah (2019) Adsorption kinetics and modeling of H2S by treated waste oil fly ash. J Air Waste Manag Assoc 69:246–257. https://doi.org/10.1080/10962247.2018.1536004
Ukanwa KS, Patchigolla K, Sakrabani R, Anthony E, Mandavgane S (2019) A review of chemicals to produce activated carbon from agricultural waste biomass. Sustain 11:1–35. https://doi.org/10.3390/su11226204
Oginni O, Singh K, Oporto G, Dawson-Andoh B, McDonald L, Sabolsky E (2019) Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour Technol Reports 7:100266. https://doi.org/10.1016/j.biteb.2019.100266
Ahmed MB, Hasan Johir MA, Zhou JL, Ngo HH, Nghiem LD, Richardson C, Moni MA, Bryant MR (2019) Activated carbon preparation from biomass feedstock: clean production and carbon dioxide adsorption. J Clean Prod 225:405–413. https://doi.org/10.1016/j.jclepro.2019.03.342
Shawabkeh RA, Aslam Z, Hussien IA (2015) Thermochemical treatment of fly ash for synthesis of mesoporous activated carbon. J Therm Anal Calorim 122:1191–1201. https://doi.org/10.1007/s10973-015-4964-7
Veeramani V, Sivakumar M, Chen SM, Madhu R, Alamri HR, Alothman ZA, Hossain MSA, Chen CK, Yamauchi Y, Miyamoto N, Wu KCW (2017) Lignocellulosic biomass-derived, graphene sheet-like porous activated carbon for electrochemical supercapacitor and catechin sensing. RSC Adv 7:45668–45675. https://doi.org/10.1039/c7ra07810b
Contescu C, Adhikari S, Gallego N, Evans N, Biss B (2018) Activated carbons derived from high-temperature pyrolysis of lignocellulosic biomass. J Carbon Res 4:1–16. https://doi.org/10.3390/c4030051
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sust Energ Rev 82:1393–1414. https://doi.org/10.1016/j.rser.2017.04.117
Zahir A, Aslam Z, Aslam U, et al (2020) Paspalum notatum grass-waste-based adsorbent for rhodamine B removal from polluted water. Chem Biochem Eng Q 34:93–104. https://doi.org/10.15255/CABEQ.2020.1830
Zhao C, Fan X, Hou X, Zhu Y, Yue Y, Wu J (2017) Extended light exposure increases stem digestibility and biomass production of switchgrass. PLoS One 12:1–17. https://doi.org/10.1371/journal.pone.0188349
Mohapatra S, Samparana S, Prerna M, Hrudayanath B (2019) Engineering grass biomass for sustainable and enhanced bioethanol production. Planta 250:395–412. https://doi.org/10.1007/s00425-019-03218-y
Xu F, Yu J, Tesso T, Dowell F, Wang D (2013) Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques : a mini-review. Appl Energy 104:801–809. https://doi.org/10.1016/j.apenergy.2012.12.019
Gil M, Pasieczna-Patkowska S, Nowicki P (2019) Application of microwave heating in the preparation of functionalized activated carbons. Adsorption 25:327–336. https://doi.org/10.1007/s10450-019-00017-5
Jawad AH, Ismail K, Ishak MAM, Wilson LD (2019) Conversion of Malaysian low-rank coal to mesoporous activated carbon: structure characterization and adsorption properties. Chinese J Chem Eng 27:1716–1727. https://doi.org/10.1016/j.cjche.2018.12.006
Wu S, He H, Li X, Yang C, Zeng G, Wu B, He S, Lu L (2018) Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: performances and mechanisms. Chem Eng J 341:126–136. https://doi.org/10.1016/j.cej.2018.01.136
Das A, Rahimi A, Ulbrich A, Alherech M, Motagamwala AH, Bhalla A, da Costa Sousa L, Balan V, Dumesic JA, Hegg EL, Dale BE, Ralph J, Coon JJ, Stahl SS (2018) Lignin conversion to low-molecular-weight aromatics via an aerobic oxidation-hydrolysis sequence: comparison of different lignin sources. ACS Sustain Chem Eng 6:3367–3374. https://doi.org/10.1021/acssuschemeng.7b03541
Deuss PJ, Scott M, Tran F, Westwood NJ, de Vries JG, Barta K (2015) Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J Am Chem Soc 137:7456–7467. https://doi.org/10.1021/jacs.5b03693
Cazetta AL, Vargas AMM, Nogami EM, Kunita MH, Guilherme MR, Martins AC, Silva TL, Moraes JCG, Almeida VC (2011) NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J 174:117–125. https://doi.org/10.1016/j.cej.2011.08.058
Su F, Lu C, Hu S (2010) Adsorption of benzene, toluene, ethylbenzene and p-xylene by NaOCl-oxidized carbon nanotubes. Colloids Surfaces A Physicochem Eng Asp 353:83–91. https://doi.org/10.1016/j.colsurfa.2009.10.025
Carrier M, Loppinet-serani A, Denux D, Lasnier J (2011) Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 35:298–307. https://doi.org/10.1016/j.biombioe.2010.08.067
Sait HH, Hussain A, Adam A, Nasir F (2012) Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour Technol 118:382–389. https://doi.org/10.1016/j.biortech.2012.04.081
Villaseñor J, Sánchez P, Valverde JL (2012) Thermogravimetric – mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour Technol 109:163–172. https://doi.org/10.1016/j.biortech.2012.01.001
Sanchez-Silva L, López-González D, Villaseñor J, Sánchez P, Valverde JL (2012) Thermogravimetric-mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour Technol 109:163–172. https://doi.org/10.1016/j.biortech.2012.01.001
Zahir A, Aslam Z, Kamal MS, Ahmad W, Abbas A, Shawabkeh RA (2017) Development of novel cross-linked chitosan for the removal of anionic Congo red dye. J Mol Liq 244:211–218. https://doi.org/10.1016/j.molliq.2017.09.006
Kumar A, Jena HM (2015) High surface area microporous activated carbons prepared from fox nut (Euryale ferox) shell by zinc chloride activation. Appl Surf Sci 356:753–761. https://doi.org/10.1016/j.apsusc.2015.08.074
Claoston N, Samsuri AW, Ahmad Husni MH, Mohd Amran MS (2014) Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag Res 32:331–339. https://doi.org/10.1177/0734242X14525822
Keiluweit M, Nico PS, Johnson MG (2010) Dynamic molecular structure of plant biomass-derived black carbon(biochar). Environ Sci Technol 44:1247–1253. https://doi.org/10.1021/es9031419
Pereira SC, Maehara L, Machado CMM, Farinas CS (2016) Physical-chemical-morphological characterization of the whole sugarcane lignocellulosic biomass used for 2G ethanol production by spectroscopy and microscopy techniques. Renew Energy 87:607–617. https://doi.org/10.1016/j.renene.2015.10.054
Tan H, Wang S (2010) Experimental study of the effect of acid-washing pretreatment on biomass pyrolysis. J Fuel Chem Technol 37:668–672. https://doi.org/10.1016/s1872-5813(10)60014-x
Contescu CI, Adhikari SP, Gallego NC, Evans N, Biss B (2018) Activated carbons derived from high-temperature pyrolysis of lignocellulosic biomass. J Carbon Res 4:1–16. https://doi.org/10.3390/c4030051
Mohamad Nor N, Lau LC, Lee KT, Mohamed AR (2013) Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control - a review. J Environ Chem Eng 1:658–666. https://doi.org/10.1016/j.jece.2013.09.017
Ioannidou O, Zabaniotou A (2007) Agricultural residues as precursors for activated carbon production-a review. Renew Sust Energ Rev 11:1966–2005. https://doi.org/10.1016/j.rser.2006.03.013
Li M, Xiao R (2019) Preparation of a dual pore structure activated carbon from rice husk char as an adsorbent for CO2 capture. Fuel Process Technol 186:35–39. https://doi.org/10.1016/j.fuproc.2018.12.015
Kim MJ, Choi SW, Kim H, Mun S, Lee KB (2020) Simple synthesis of spent coffee ground-based microporous carbons using K2CO3 as an activation agent and their application to CO2 capture. Chem Eng J 397:125404. https://doi.org/10.1016/j.cej.2020.125404
Teague CM, Schott JA, Stieber C, Mann ZE, Zhang P, Williamson BR, Dai S, Mahurin SM (2019) Microporous and hollow carbon spheres derived from soft drinks: promising CO2 separation materials. Microporous Mesoporous Mater 286:199–206. https://doi.org/10.1016/j.micromeso.2019.04.017
Huang GG, Liu YF, Wu XX, Cai JJ (2019) Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. Xinxing Tan Cailiao/New Carbon Mater 34:247–257. https://doi.org/10.1016/S1872-5805(19)60014-4
Heo YJ, Park SJ (2015) A role of steam activation on CO2 capture and separation of narrow microporous carbons produced from cellulose fibers. Energy 91:142–150. https://doi.org/10.1016/j.energy.2015.08.033
Yang H, Gong M, Chen Y (2011) Preparation of activated carbons and their adsorption properties for greenhouse gases: CH4 and CO2. J Nat Gas Chem 20:460–464. https://doi.org/10.1016/S1003-9953(10)60232-0
Chiang YC, Hsu WL, Lin SY, Juang RS (2017) Enhanced CO2 adsorption on activated carbon fibers grafted with nitrogen-doped carbon nanotubes. Materials (Basel) 10:1–12. https://doi.org/10.3390/ma10050511
Acknowledgments
Authors acknowledge the Department of Chemical Engineering, University of Engineering and Technology, Lahore, Pakistan, for providing the funding and facilities to conduct this research.
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslam, Z., Anait, U., Abbas, A. et al. Adsorption of carbon dioxide onto activated carbon prepared from lawn grass. Biomass Conv. Bioref. 12, 3121–3131 (2022). https://doi.org/10.1007/s13399-020-01029-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01029-w