Abstract
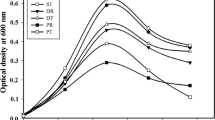
The aim of the present study was to generate a consistent data set for biorefinery intermediates from novel energy crop Silphium perfoliatum compared with Fagus sylvatica, including process streams from liquid hot water pretreatment, enzymatic hydrolysis, and fermentation with Bacillus licheniformis. The consistency of the data set was further supported by the application of a consistent analytical method based on high performance anion exchange chromatography with pulsed amperometric detection and validated for process intermediates, which renders the technique a versatile analytical tool also for alcoholic compounds. For the first time, Silphium perfoliatum was used for liquid hot water pretreatment which resulted in a maximal absolute glucose recovery after enzymatic hydrolysis for feedstock pretreated with 200 °C, 20 min, 20% solid loading. Under these conditions, 68% glucose were recovered for Silphium perfoliatum and 62% for Fagus sylvatica. Further, enzymatic hydrolyzates of both feedstocks were successfully used as single carbon sources for 2,3-butanediol fermentation with Bacillus licheniformis resulting in a 2,3-butanediol yield of 39% and 31% of the theoretical yield for Silphium perfoliatum and Fagus sylvatica, respectively. Thus, the technical suitability of Silphium perfoliatum as feedstock for a liquid hot water–based biorefinery process was comprehensively demonstrated and successfully bore the comparison with Fagus sylvatica.





Similar content being viewed by others
Data Availability
Not applicable
References
Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA (2007) Climate change 2007: mitigation of the climate change. Cambridge University Press www.ipcc.ch/site/assets/uploads/2018/03/ar4_wg3_full_report-1.pdf. Accessed 23 August 2020
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K, Tignor MMB, Miller HL (2007) Climate change 2007: the physical science basis. Cambridge University Press www.ipcc.ch/site/assets/uploads/2018/05/ar4_wg1_full_report-1.pdf. Accessed 23 August 2020
van Wyk JP (2001) Biotechnology and the utilization of biowaste as a resource for bioproduct development. 5. Trends Biotechnol 19:172–177. https://doi.org/10.1016/S0167-7799(01)01601-8
Neumerkel W, Martin B (1982) Silphium (Silphium perfoliatum L) - a new feed plant. Arch Acker Pfl Boden 26:261–271
Gansberger M, Montgomery LFR, Liebhard P (2015) Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: a review. Ind Crop Prod 63:362–372. https://doi.org/10.1016/j.indcrop.2014.09.047
Wever C, Höller M, Becker L, Biertümpfel A, Köhler J, van Inghelandt D, Westhoff P, Pude R, Pestsova E (2019) Towards high-biomass yielding bioenergy crop Silphium perfoliatum L.: phenotypic and genotypic evaluation of five cultivated populations. Biomass Bioenergy 124:102–113. https://doi.org/10.1016/j.biombioe.2019.03.016
Sokolov VS, Gritsak ZI (1972) Silphium – a valuable fodder and nectariferous crop. World Crops 24(6):299–301
Deim F, Mayr J, Liebhard P (2014) Silphium Perfoliatum L. - eine Alternative in der Produktion nachwachsender Rohstoffe in Österreich. Mitt Ges Pflanzenbauwiss 26:114–115
Tomaszewska-Sowa M, Figas A (2011) Optimisation of the processes of sterilization and micropropagation of cup plants (Silphium perfoliatum L.) from apical explants of seedlings in in vitro cultures. Acta Agrobot 64(4):3–10. https://doi.org/10.5586/aa.2011.041
Mosier NS, Wyman C, Dale B, Elander R, Lee Y, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686. https://doi.org/10.1016/j.biortech.2004.06.025
Pérez JA, Ballesteros I, Ballesteros M, Sáez F, Negro MJ, Manzanares P (2008) Optimizing liquid hot water pretreatment conditions to enhance monosaccharide recovery from wheat straw for fuel-ethanol production. Fuel 87(17):3640–3647. https://doi.org/10.1016/j.fuel.2008.06.009
Pedersen M, Viksø-Nielsen A, Meyer AS (2010) Monosaccharide yields and lignin removal from wheat straw in response to catalyst type and pH during mild thermal pretreatment. Process Biochem 45(7):1181–1186. https://doi.org/10.1016/j.procbio.2010.03.020
Ruiz HA, Conrad M, Sun S-N, Sanchez A, Rocha GJM, Romaní A, Castro E, Torres A, Rodríguez-Jasso RM, Andrade LP, Smirnova I, Sun R-C, Meyer AS (2019) Engineering aspects of hydrothermal pretreatment: from batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour Technol 299:122685. https://doi.org/10.1016/j.biortech.2019.122685
Ruiz HA, Silva DP, Ruzene DS, Lima LF, Vicente AA, Teixeira JA (2012) Bioethanol production from hydrothermal pretreated wheat straw by a flocculating Saccharomyces cerevisiae strain – effect of process conditions. Fuel 95:528–536. https://doi.org/10.1016/j.fuel.2011.10.060
Ruiz HA, Rodríguez-Jasso RM, Fernandes BD, Vicente AA, Teixeira JA (2013) Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review. Renew Sust Energ Rev 21:35–51. https://doi.org/10.1016/j.rser.2012.11.069
Sun S-L, Sun S-N, Wen J-L, Zhang X-M, Peng F, Sun R-C (2015) Assessment of integrated process based on hydrothermal and alkaline treatments for enzymatic saccharification of sweet sorghum stems. Bioresour Technol 175:473–479. https://doi.org/10.1016/j.biortech.2014.10.111
Xiao X, Bian J, Li M-F, Xu H, Xiao B, Sun RC (2014) Enhanced enzymatic hydrolysis of bamboo (Dendrocalamus giganteus Munro) culm by hydrothermal pretreatment. Bioresour Technol 159:41–47. https://doi.org/10.1016/j.biortech.2014.02.096
Li M, Cao S, Meng X, Studer M, Wyman CE, Ragauskas AJ, Pu Y (2017) The effect of liquid hot water pretreatment on the chemical–structural alteration and the reduced recalcitrance in poplar. Biotechnol Biofuels 10(1):237. https://doi.org/10.1186/s13068-017-0926-6
Ma XJ, Cao SL, Lin L, Luo XL, Hu HC, Chen LH, Huang LL (2013) Hydrothermal pretreatment of bamboo and cellulose degradation. Bioresour Technol 148:408–413. https://doi.org/10.1016/j.biortech.2013.09.021
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685. https://doi.org/10.1016/j.biotechadv.2011.05.005
Wang Z-W, Zhu M-Q, Li M-F, Wang J-Q, Wei Q, Sun R-C (2016) Comprehensive evaluation of the liquid fraction during the hydrothermal treatment of rapeseed straw. Biotechnol Biofuels 9(1):142. https://doi.org/10.1186/s13068-016-0552-8
Chen X, Li H, Sun S, Cao X, Sun R (2018) Co-production of oligosaccharides and fermentable sugar from wheat straw by hydrothermal pretreatment combined with alkaline ethanol extraction. Ind Crop Prod 111:78–85. https://doi.org/10.1016/j.indcrop.2017.10.014
Pu Y, Hu F, Huang F, Davison BH, Ragauskas AJ (2013) Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol Biofuels 6:15. https://doi.org/10.1186/1754-6834-6-15
Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2008) Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol Bioeng 101(5):913–925. https://doi.org/10.1002/bit.21959
Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2007) Deposition of lignin droplets produced during dilute acid pretreatment of maize stems retards enzymatic hydrolysis of cellulose. Biotechnol Prog 23(6):1333–1339. https://doi.org/10.1021/bp0702018
Ruiz HA, Ruzene DS, Silva DP, Quintas MAC, Vicente AA, Teixeira JA (2010) Evaluation of a hydrothermal process for pretreatment of wheat straw-effect of particle size and process conditions. J Chem Technol Biotechnol 86(1):88–94. https://doi.org/10.1002/jctb.2518
Hu F, Jung S, Ragauskas A (2012) Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour Technol 117:7–12. https://doi.org/10.1016/j.biortech.2012.04.037
Pino MS, Rodríguez-Jasso RM, Michelin M, Ruiz HA (2019) Enhancement and modeling of enzymatic hydrolysis on cellulose from Agave bagasse hydrothermally pretreated in a horizontal bioreactor. Carbohydr Polym 211:349–359. https://doi.org/10.1016/j.carbpol.2019.01.111
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109(4):1083–1087. https://doi.org/10.1002/bit.24370
Kim S, Dale BE (2004) Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 26(4):361–375. https://doi.org/10.1016/j.biombioe.2003.08.002
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem 12(4):539–554. https://doi.org/10.1039/b922014c
Jang Y, Kim B, Shin JH, Choi YJ, Choi S, Song CW, Lee J, Park GH, Lee YS (2012) Bio-based production of C2–C6 platform chemicals. Biotechnol Bioeng 109(10):2437–2459. https://doi.org/10.1002/bit.24599
Häßler T, Schieder D, Pfaller R, Faulstich M, Sieber V (2012) Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour Technol 124:237–244. https://doi.org/10.1016/j.biortech.2012.08.047
Ma K, He M, You H, Pan L, Wanga Z, Wang Y, Hu G, Cui Y, Maeda T (2018) Improvement of (R,R)-2,3-butanediol production from corn stover hydrolysate by cell recycling continuous fermentation. Chem Eng J 332:361–369. https://doi.org/10.1016/j.cej.2017.09.097
Hazeena SH, Salini NC, Sindhu R, Pandey A, Binod P (2019) Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour Technol 278:145–149. https://doi.org/10.1016/j.biortech.2019.01.042
Jurchescu I, Hamann J, Zhou X, Ortmann T, Kuenz A, Prüße U, Lang S (2013) Enhanced 2,3-butanediol production in fed-batch cultures of free and immobilized Bacillus licheniformis DSM 8785. Appl Microbiol Biotechnol 97(15):6715–6723. https://doi.org/10.1007/s00253-013-4981-z
Celińska E, Grajek W (2009) Biotechnological production of 2,3-butanediol—current state and prospects. Biotechnol Adv 27(6):715–725. https://doi.org/10.1016/j.biotechadv.2009.05.002
Xiao Z, Lu JR (2014) Strategies for enhancing fermentative production of acetoin: a review. Biotechnol Adv 32(2):492–503. https://doi.org/10.1016/j.biotechadv.2014.01.002
Song K, Chu Q, Hu J, Bu Q, Li F, Chen X, Shi A (2019) Two-stage alkali-oxygen pretreatment capable of improving biomass saccharification for bioethanol production and enabling lignin valorization via adsorbents for heavy metal ions under the biorefinery concept. Bioresour Technol 276:161–169. https://doi.org/10.1016/j.biortech.2018.12.107
Penín L, Peleteiro S, Santos V, Alonso JL, Parajo JC (2018) Selective fractionation and enzymatic hydrolysis of Eucalyptus nitens wood. Cellulose 26(2):1125–1139. https://doi.org/10.1007/s10570-018-2109-4
Xing Y, Bu L, Zheng T, Liu S, Jiang J (2016) Enhancement of high-solids enzymatic hydrolysis of corncob residues by bisulfite pretreatment for biorefinery. Bioresour Technol 221:461–468. https://doi.org/10.1016/j.biortech.2016.09.086
Viell J, Wulfhorst H, Schmidt T, Commandeur U, Fischer R, Spiess AC, Marquardt W (2013) An efficient process for the saccharification of wood chips by combined ionic liquid pretreatment and enzymatic hydrolysis. Bioresour Technol 146:144–151. https://doi.org/10.1016/j.biortech.2013.07.059
Regestein L, Klement T, Grande P, Kreyenschulte D, Heyman B, Maßmann T, Eggert A, Sengpiel R, Wang Y, Wierckx N, Blank LM, Spiess AC, Leitner W, Bolm C, Wessling M, Jupke A, Rosenbaum M, Büchs J (2018) From beech wood to itaconic acid: case study on biorefinery process integration. Biotechnol Biofuels 11:279. https://doi.org/10.1186/s13068-018-1273-y
Grande PM, Viell J, Theyssen N, Marquardt W, Domínguez de María P, Leitner W (2015) Fractionation of lignocellulosic biomass using the OrganoCat process. Green Chem 17(6):3533–3539. https://doi.org/10.1039/c4gc02534b
Garrote G, Domínguez H, Parajó JC (1999) Mild autohydrolysis: an environmentally friendly technology for xylooligosaccharide production from wood. J Chem Technol Biotechnol 74(11):1101–1109. https://doi.org/10.1002/(SICI)1097-4660(199911)74:11<1101::AID-JCTB146>3.0.CO;2-M
Laure S, Leschinsky M, Fröhling M, Schultmann F, Unkelbach G (2014) Assessment of an organosolv lignocellulose biorefinery concept based on a material flow analysis of a pilot plant. Cellul Chem Technol 48(9-10):793–798
Michels J, Wagemann K (2010) The German lignocellulose feedstock biorefinery project. Biofuels Bioprod Biorefin 4(3):263–267. https://doi.org/10.1002/bbb.216
Miazek K, Remacle C, Richel A, Goffin D (2017) Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour Technol 230:122–131. https://doi.org/10.1016/j.biortech.2017.01.034
Budzinski M, Nitzsche R (2016) Comparative economic and environmental assessment of four beech wood based biorefinery concepts. Bioresour Technol 216:613–621. https://doi.org/10.1016/j.biortech.2016.05.111
Dieter M, Englert H, Klein M (2001) Abschätzung des Rohholzpotentials für die energetische Nutzung in der Bundesrepublik Deutschland. Bundesforschungsanstalt für Forst- und Holzwirtschaft, Institut für Ökonomie. literatur.thuenen.de/digbib_extern/bitv/dk040192.pdf. Accessed 24 August 2020
Luo G, Talebnia F, Karakashev D, Xie L, Zhou Q, Angelidaki I (2011) Enhanced bioenergy recovery from rapeseed plant in a biorefinery concept. Bioresour Technol 102:1433–1439. https://doi.org/10.1016/j.biortech.2010.09.071
Kaparaju P, Serrano M, Thomsen AB, Kongjan P, Angelidaki I (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour Technol 100:2562–2568. https://doi.org/10.1016/j.biortech.2008.11.011
Klinke HB, Ahring BK, Schmidt AS, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol 82(1):15–26. https://doi.org/10.1016/S0960-8524(01)00152-3
Anders N, Humann H, Langhans B, Spiess AC (2015) Simultaneous determination of acid-soluble biomass-derived compounds using high performance anion exchange chromatography coupled with pulsed amperometric detection. Anal Methods 7:7866–7873. https://doi.org/10.1039/C5AY01371B
Épshtein NA (2004) Validation of HPLC techniques for pharmaceutical analysis. Pharm Chem J 38(4):212–228. https://doi.org/10.1023/B:PHAC.0000038422.27193.6c
Sluiter A, Hamas B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedures, Technical Report NREL/TP-510–42618. www.nrel.gov/docs/gen/fy13/42618.pdf. Accessed 24 August 2020
Overend RP, Chornet E (1987) Fractionation of lignocellulosics by steamaqueous pretreatments. Philos Trans R Soc London Ser A 321:523–536. https://doi.org/10.1098/rsta.1987.0029
Adney B, Baker J (1996) Measurement of cellulase activities. Laboratory Analytical Procedure, Technical Report NREL/TP-510-42628. www.nrel.gov/docs/gen/fy08/42628.pdf. Accessed 24 August 2020
Nakashimada Y, Kanai K, Nishio N (1998) Optimization of dilution rate, pH and oxygen supply on optical purity of 2, 3-butanediol produced by Paenibacillus polymyxa in chemostat culture. Biotechnol Lett 20(12):1133–1138. https://doi.org/10.1023/A:1005324403186
Heyman B, Lamm R, Tulke H, Regestein L, Büchs J (2019) Shake flask methodology for assessing the influence of the maximum oxygen transfer capacity on 2,3-butanediol production. Microb Cell Factories 18:78. https://doi.org/10.1186/s12934-019-1126-9
Heyman B, Tulke H, Putri SP, Fukusaki E, Büchs J (2020) Online monitoring of the respiratory quotient reveals metabolic phases during microaerobic 2,3-butanediol production with Bacillus licheniformis. Eng Life Sci 20:133–144. https://doi.org/10.1002/elsc.201900121
Wandrey GB (2017) Light-mediated control and analysis of recombinant protein production in microscale cultivations. Dissertation, RWTH Aachen University
Cürten C, Anders N, Juchem N, Ihling N, Volkenborn K, Knapp A, Jaeger K, Büchs J, Spiess AC (2018) Fast automated online xylanase activity assay using HPAEC-PAD. Anal Bioanal Chem 410(1):57–69. https://doi.org/10.1007/s00216-017-0712-0
Anders N, Schelden M, Roth S, Spiess AC (2017) Automated chromatographic laccase-mediator-system activity assay. Anal Bioanal Chem 409(20):4801–4809. https://doi.org/10.1007/s00216-017-0423-6
Schulz M, Filary B, Kühn S, Colby T, Harzen A, Schmidt J, Sicker D, Hennig L, Hofmann D, Disko U, Anders N (2016) Benzoxazolinone detoxification by N-glucosylation: the multi-compartment-network of Zea mays L. Plant Signal Behav 11(1):e1119962. https://doi.org/10.1080/15592324.2015.1119962
Hanko VP, Rohrer JS (2000) Determination of carbohydrates, sugar alcohols, and glycols in cell cultures and fermentation broths using high-performance anion-exchange chromatography with pulsed amperometric detection. Anal Biochem 283(2):192–199. https://doi.org/10.1006/abio.2000.4653
Chang KC, Dhurandhar N, You X, Miyamoto A (1994) Cultivar/location and processing methods affect yield and quality of sunflower pectin. J Food Sci 59(3):602–605. https://doi.org/10.1111/j.1365-2621.1994.tb05572.x
Miyamoto A, Chang KC (1992) Extraction and physicochemical characterization of pectin from sunflower head residues. J Food Sci 57(6):1439–1443. https://doi.org/10.1111/j.1365-2621.1992.tb06878.x
Leitao MCA, Alado Silva ML, Januiriob MIN, Azinheira HG (1995) Galacturonic acid in pectic substances of sunflower head residues: quantitative determination by HPLC. Carbohydr Polym 26(3):165–169. https://doi.org/10.1016/0144-8617(95)00003-P
Jing Q, Lü X (2008) Kinetics of non-catalyzed decomposition of glucose in high-temperature liquid water. Chin J Chem Eng 16(6):890–894. https://doi.org/10.1016/S1004-9541(09)60012-4
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant N (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym Microb Technol 24(3-4):151–159. https://doi.org/10.1016/S0141-0229(98)00101-X
Thomasser C, Danner H, Neureiter M, Saidi B, Braun R (2002) Thermophilic fermentation of hydrolysates – the effect of inhibitors on growth of thermophilic bacteria. Appl Biochem Biotechnol 98-100(1-9):765–773. https://doi.org/10.1385/ABAB:98-100:1-9:765
Fang Q, Hanna MA (2002) Experimental studies for levulinic acid production from whole kernel grain sorghum. Bioresour Technol 81(3):187–192. https://doi.org/10.1016/S0960-8524(01)00144-4
Mussatto SI, Fernandes M, Milagres AMF, Roberto IC (2008) Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzym Microb Technol 43(2):124–129. https://doi.org/10.1016/j.enzmictec.2007.11.006
Vázquez G, Antorrena G, Gonzfilez J, Freire S, López S (1997) Acetosolv pulping of pine wood. Kinetic modelling of lignin solubilization and condensation. Bioresour Technol 59(2-3):121–127. https://doi.org/10.1016/S0960-8524(96)00168-X
Parajo LC, Alonso JL, Santos V (1995) Kinetics of catalyzed organosolv processing of pine wood. Ind Eng Chem Res 34:4333–4342. https://doi.org/10.1021/ie00039a025
Zhang X, Zhu J, Sun L, Yuan Q, Cheng G, Argyropoulos DS (2019) Extraction and characterization of lignin from corncob residue after acid-catalyzed steam explosion pretreatment. Ind Crop Prod 133:241–249. https://doi.org/10.1016/j.indcrop.2019.03.027
Author not applicable (2007) Complex carbohydrate analysis: enzymes, kits and reagents. Sigma-Aldrich 2(3). www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/General_Information/carbohydrate_analysis_bf8.pdf. Accessed 12 December 2019
Author not applicable (2017) Enzyme product brochure. Megazyme. www.megazyme.com/media/pdf/77/12/18/enzyme-product-guide_compressed.pdf. Accessed 12 December 2019
Chernoglazov VM, Ermolova OV, Klyosov AA (1988) Adsorption of high-purity endo-1,4-b-glucanases from Trichoderma reesei on components of lignocellulosic materials: cellulose, lignin, and xylan. Enzym Microb Technol 10(8):503–507. https://doi.org/10.1016/0141-0229(88)90029-4
Converse AO, Ooshima H, Burns DS (1990) Kinetics of enzymatic hydrolysis of lignocellulosic materials based on surface area of cellulose accessible to enzyme and enzyme adsorption on lignin and cellulose. Appl Biochem Biotechnol 24:67–73. https://doi.org/10.1007/BF02920234
Ooshima H, Burns D, Converse AO (1990) Adsorption of cellulase from Trichoderma reesei on cellulose and lignacious residue in wood pretreated by dilute sulfuric acid with explosive decompression. Biotechnol Bioeng 36(5):446–452. https://doi.org/10.1002/bit.260360503
Mooney CA, Mansfield SD, Touhy MG, Saddler JN (1998) The effect of initial pore volume and lignin content of the enzymatic hydrolysis of softwoods. Bioresour Technol 64(2):113–119. https://doi.org/10.1016/S0960-8524(97)00181-8
Modenbach AA, Nokes SE (2013) Enzymatic hydrolysis of biomass at high-solids loadings – a review. Biomass Bioenergy 56:526–544. https://doi.org/10.1016/j.biombioe.2013.05.031
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefin 1:119–134. https://doi.org/10.1002/bbb.4
Larsen J, Petersen MO, Thirup L, Li HW, Iversen FK (2008) The IBUS process-lignocellulosic bioethanol close to a commercial reality. Chem Eng Technol 31(5):765–772. https://doi.org/10.1002/ceat.200800048
Qing Q, Yang B, Wyman CE (2010) Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour Technol 101(24):9624–9630. https://doi.org/10.1016/j.biortech.2010.06.137
Damm T, Grande PM, Jablonowski ND, Thiele B, Disko U, Mann U, Schurr U, Leitner W, Usadel B, Domínguez de María P, Klose H (2017) OrganoCat pretreatment of perennial plants: synergies between a biogenic fractionation and valuable feedstocks. Bioresour Technol 244(1):889–896. https://doi.org/10.1016/j.biortech.2017.08.027
Voloch M, Jansen NB, Ladisch MR, Tsao GT, Narayan R, Rodwell VW (1985) 2,3- Butanediol. In: Moo-Young M (ed) Comprehensive biotechnoiogy: the principles, applications and regulations of biotechnology in industry, agriculture and medicine. Pergamon, Oxford, pp 933–947
Guragaina YN, Chittab D, Karanjikarc M, Vadlania PV (2017) Appropriate lignocellulosic biomass processing strategies for efficient 2,3-butanediol production from biomass-derived sugars using Bacillus licheniformis DSM 8785. Food Bioprod Process 104:147–158. https://doi.org/10.1016/j.fbp.2017.05.010
Klement T, Milker S, Jäger G, Grande PM, Domínguez de María P, Büchs J (2012) Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb Cell Factories 11:43. https://doi.org/10.1186/1475-2859-11-43
Converti A, Perego P, Del Borghi M (2003) Effect of specific oxygen uptake rate on Enterobacter aerogenes energetics: carbon and reduction degree balances in batch cultivations. Biotechnol Bioeng 82:370–377. https://doi.org/10.1002/bit.10570
Rebecchi S, Pinelli D, Zanaroli G, Fava F, Frascari D (2018) Effect of oxygen mass transfer rate on the production of 2,3-butanediol from glucose and agro-industrial byproducts by Bacillus licheniformis ATCC9789. Biotechnol Biofuels 11:145. https://doi.org/10.1186/s13068-018-1138-4
Kim Y, Mosier NS, Ladisch MR (2009) Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnol Prog 25(2):340–348. https://doi.org/10.1002/btpr.137
Aguilar DL, Rodríguez-Jasso RM, Zanuso E, de Rodríguez DJ, Amaya-Delgado L, Sanchez A, Ruiz HA (2018) Scale-up and evaluation of hydrothermal pretreatment in isothermal and non-isothermal regimen for bioethanol production using agave bagasse. Bioresour Technol 263:112–119. https://doi.org/10.1016/j.biortech.2018.04.100
Ko JK, Kim Y, Ximenes E, Ladisch MR (2014) Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol Bioeng 112(2):252–262. https://doi.org/10.1002/bit.25349
Wang Q, Chen T, Zhao X, Chamu J (2012) Metabolic engineering of thermophilic Bacillus licheniformis for chiral pure D-2,3-butanediol production. Biotechnol Bioeng 109(7):1610–1621. https://doi.org/10.1002/bit.24427
Moes J, Griot M, Keller J, Heinzle E, Dunn IJ, Bourne JR (1985) A microbial culture with oxygen-sensitive product distribution as a potential tool for characterizing bioreactor oxygen transport. Biotechnol Bioeng 27(4):482–489. https://doi.org/10.1002/bit.260270413
Acknowledgments
This study was part of the Silphium Perfoliatum Resource Evaluation And Development (SPREAD) project and was financially supported by the Bioeconomic science center (BioSC). The BioSC is supported by the state of North Rhine-Westphalia (NRW), Germany, on a long-term basis within the framework of the NRW-Strategieprojekt BioSC. Further, a section of this work was performed as part of the Cluster of Excellence “Tailor-Made Fuels from Biomass” (TMFB), which is funded by the Excellence Initiative of the German federal and state governments to promote science and research at German universities. A part of this study was performed within the project “Exchange for Teaching and Research between Aachen and Tunis” (Ex-TRAcT), in cooperation with the Private University of Tunis (Tunisia), and was founded by the program German-Arab short-term measures as part of the German-Arab Transformation Partnership of the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Code Availability
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 294 kb).
Rights and permissions
About this article
Cite this article
Lunze, A., Heyman, B., Chammakhi, Y. et al. Investigation of Silphium perfoliatum as Feedstock for a Liquid Hot Water–Based Biorefinery Process Towards 2,3-Butanediol. Bioenerg. Res. 14, 799–814 (2021). https://doi.org/10.1007/s12155-020-10194-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10194-9