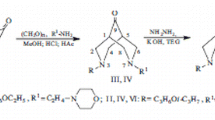
Simultaneous Mannich condensation of 1-(3-methoxypropyl)piperidone-4 with paraformaldehyde and 1-(2-pyridinoethyl)amine yielded 3-(3methoxypropyl)-7-[2-(pyridin-2-yl)ethyl]-3,7-diazabicyclo[3.3.1]nonan-9-one, which was reduced to the corresponding bicyclic nonane under Huang—Minlon reaction conditions. The reaction of 3,7-diazabicyclo[3.3.1]nonan-9-one with hydroxylamine hydrochloride led to the corresponding oxime, subsequent acylation of which synthesized its O-benzoyloxime. Replacement of the morpholine or piperazine ring in 3-(3-ethoxy- or isopropoxypropyl)-7-[2-(N-morpholino- or N-piperazino) ethyl]-3,7-diazabicyclo[3.3.1]nonane by pyridine led to a loss of activity. However, 3-(3-methoxy- and isopropoxypropyl)-7-[2-(pyridin-2-yl- and N-piperazino)ethyl]-3,7-diazabicyclo[3.3.1]nonan-9-one O-benzoyloximes were myelostimulators although they differed in that the β-cyclodextrin complex of 3-(3-methoxypropyl)-7-[2-(pyridin-2-yl)ethyl]-3,7-diazabicyclo[3.3.1]nonan-9-one O-benzoyloxime stimulated both erythro- and leukopoiesis while its N-piperazine analog stimulated only leukopoiesis.


Similar content being viewed by others
References
A. L. Lefebvre and L. McAuliffe, R. I. Med. J., 99(12), 19 – 22 (2016).
A. Mendes and M. J. Sa, Arq. Neuro-Psiquiatr., 69(3), 536 – 543 (2011).
Rep. Kazakhstan Pat. No. 14323, Oct. 28, 2002; Byull. Izobret., 5 (2004).
T. K. Iskakova, S. N. Shin, N. A. Zhumanova, et al., in: Proceedings of the International Conference “Current State and Prospects for Development of Organic Chemistry in the Republic” Dedicated to the 90th Birthday of I. N. Azerbaev [in Russian], Shymkent, 2002, pp. 155 – 158.
T. K. Iskakova, Izv. Nauchno-Tekh. O-va. Kakhak, 2, 52 – 56 (2007).
T. K. Iskakova, in: Proceedings of the VIth International Beremzhanovskii Convention on Chemistry and Chemical Engineering [in Russian], Karaganda, 2008, pp. 370 – 373.
G. S. Smagulova, K. D. Praliev, et al., Rep. Kazakhstan Pat. No. 2464, Mar. 9, 2017; Byull. Izobret., 21 (2017).
V. K. Yu, T. K. Iskakova, et al., Rep. Kazakhstan Pat. No. 2413, Oct. 27, 2016; Byull Izobret., 19 (2017).
Ye. B. Tolisbaev, N. A. Togyzbaeva, A. Ye. Malmakova, et al., in: IVth International Conference “Innovative Ideas and Technologies-2011,” Almaty, 2011, pp. 229 – 230.
K. D. Praliev, T. K. Iskakova, L. K. Baktybaeva, and A. E. Malmakova, Khim.-farm. Zh., 49(5), 8 – 11 (2015); Pharm. Chem. J., 49(5), 292 – 295 (2015).
L. A. Chaika, Ya. I. Khadzhai, and V. V. Libina, Khim.-farm. Zh., 24(7), 19 – 22 (1990).
Handbook for Preclinical (Nonclinical) Studies of Biologically Active Compounds. Ethical Principles and Rules for Conducting Scientific Animal Experiments, Ministry of the Republic of Kazakhstan Order No. 415, May 29, 2015.
“Ethical principles and guidelines for scientific experiments on animals,” Swiss Academy of Medical Sciences and Swiss Academy of Sciences, Experientia, 48, 1 – 3 (1992).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 6, pp. 39 – 44, June, 2020.
Rights and permissions
About this article
Cite this article
Malmakova, A.E., Yu, V.K., Iskakova, T.K. et al. Synthesis and Myelostimulatory Activity of β-Cyclodextrin Complexes of 3,7-Diazabicyclo[3.3.1]Nonan-9-One Derivatives. Pharm Chem J 54, 582–587 (2020). https://doi.org/10.1007/s11094-020-02243-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02243-6