Abstract
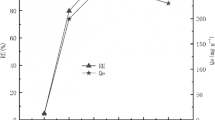
Surfactants are organic compounds which can be used in several applications. However, they can contaminate world water resources causing detrimental effects to human beings, aquatic life, and animals. This paper investigates the adsorption kinetics, isotherms, and thermodynamic properties for the removal of an anionic surfactant, sodium dodecylbenzene sulfonate (SDBS), using fly ash. Characteristics of fly ash such as surface area and pore size analysis and the point of zero charge (PZC) were determined. The effects of parameters such as pH, surfactant concentration, and temperature and the adsorption kinetics, isotherms, and thermodynamic properties and adsorption mechanism were determined. Fly ash is a mesoporous material having surface area and pore size of 1.079 m2/g and 9.813 nm and PZC at pH 6.58. pH 2 and the temperature 25 °C were optimum for adsorbing SDBS onto fly ash. The adsorption capacity and removal efficiency increased by increasing the concentration of SDBS from 100 to 2000 mg/L, indicating that the increase of surfactant concentration could not saturate the surface of fly ash. The pseudo-second-order and the Langmuir isotherm models showed best fit to the adsorption data and the thermodynamic properties described adsorption as an exothermic, barrierless, non-spontaneous, and entropy-reducing reaction which is more feasible at a lower temperature of 25 °C. This indicated that the adsorption occurs by both physisorption and chemisorption with monolayer coverage of SDBS on the surface of fly ash. SDBS surfactant adsorbed onto fly ash mainly through electrostatic interactions between oppositely charged SDBS and fly ash.





Similar content being viewed by others
Data Availability
Data can be provided on request.
References
Aboulhassan, M., Souabi, S., Yaacoubi, A., & Baudu, M. (2006). Removal of surfactant from industrial wastewaters by coagulation flocculation process. International Journal of Environmental Science and Technology, 3(4), 327–332.
Alkan, M., Karadaş, M., Doğan, M., & Demirbaş, Ö. (2005). Adsorption of CTAB onto perlite samples from aqueous solutions. Journal of Colloid and Interface Science, 291(2), 309–318.
Asakawa, T., & Ogino, K. (1986). Removal of trace organic compounds from multicomponent liquid mixtures. I. Adsorption of surfactant mixtures on activated carbon. Colloid and Polymer Science, 264(12), 1085–1089.
Bautista-Toledo, M. I., Rivera-Utrilla, J., Méndez-Díaz, J. D., Sánchez-Polo, M., & Carrasco-Marín, F. (2014). Removal of the surfactant sodium dodecylbenzenesulfonate from water by processes based on adsorption/bioadsorption and biodegradation. Journal of Colloid and Interface Science, 418, 113–119.
Beltrán-Heredia, J., Sánchez-Martín, J., & Barrado-Moreno, M. (2012). Long-chain anionic surfactants in aqueous solution. Removal by Moringa oleifera coagulant. Chemical Engineering Journal, 180, 128–136.
Boonyasuwat, S., Chavadej, S., Malakul, P., & Scamehorn, J. F. (2003). Anionic and cationic surfactant recovery from water using a multistage foam fractionator. Chemical Engineering Journal, 93(3), 241–252.
De Gisi, S., Lofrano, G., Grassi, M., & Notarnicola, M. (2016). Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustainable Materials and Technologies, 9, 10–40.
Edser, C. (2008). Status of global surfactant markets. Focus on Surfactants, 2008(11), 1–2.
Freundlich, H. (1907). Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie, 57(1), 385–470.
Gönder, Z., Vergili, I., Kaya, Y., & Barlas, H. (2010). Adsorption of cationic and anionic surfactants onto organic polymer resin Lewatit VPOC 1064 MD PH. Environmental Geochemistry and Health, 32(4), 267–273.
Gonzalez-Garcia, C., Gonzalez-Martin, M., Gallardo-Moreno, A., Gomez-Serrano, V., Labajos-Broncano, L., & Bruque, J. (2002). Removal of an ionic surfactant from wastewater by carbon blacks adsorption. Separation Science and Technology, 37(12), 2823–2837.
Gupta, S., Pal, A., Ghosh, P. K., & Bandyopadhyay, M. (2003). Performance of waste activated carbon as a low-cost adsorbent for the removal of anionic surfactant from aquatic environment. Journal of Environmental Science and Health, Part A, 38(2), 381–397.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465.
Ikehata, K., & El-Din, M. G. (2004). Degradation of recalcitrant surfactants in wastewater by ozonation and advanced oxidation processes: a review. Ozone: Science & Engineering, 26(4), 327–343.
Juang, R. S., Wu, F. C., & Tseng, R. L. (1997). The ability of activated clay for the adsorption of dyes from aqueous solutions. Environmental Technology, 18(5), 525–531.
Kahya, N., Kaygusuz, H., & Erim, F. B. (2018). Aqueous removal of sodium dodecyl benzene sulfonate (SDBS) by crosslinked chitosan films. Journal of Polymers and the Environment, 26(5), 2166–2172.
Kim, D., Kim, J., Lee, K.-W., & Lee, T. S. (2019). Removal of sodium dodecylbenzenesulfonate using surface-functionalized mesoporous silica nanoparticles. Microporous and Mesoporous Materials, 275, 270–277.
Kimerle, R. A., & Swisher, R. (1977). Reduction of aquatic toxicity of linear alkylbenzene sulfonate (LAS) by biodegradation. Water Research, 11(1), 31–37.
Kowalska, I. (2011). Ion-exchange–ultrafiltration system for surfactants removal from water solutions. Desalination and Water Treatment, 25(1–3), 47–53.
Krawczyk, J. (2018). Thermodynamic properties of disaccharide based surfactants adsorption at the water-air interface. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 551, 50–57.
Lagergren, S. (1898). About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl, 24, 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40(9), 1361–1403.
Liu, Y., Yan, C., Zhang, Z., Wang, H., Zhou, S., & Zhou, W. (2016). A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution. Fuel, 185, 181–189.
Ncibi, M. C., Gaspard, S., & Sillanpää, M. (2015). As-synthesized multi-walled carbon nanotubes for the removal of ionic and non-ionic surfactants. Journal of Hazardous Materials, 286, 195–203.
Önder, E., Koparal, A. S., & Öğütveren, Ü. B. (2007). An alternative method for the removal of surfactants from water: electrochemical coagulation. Separation and Purification Technology, 52(3), 527–532.
Pal, A., Pan, S., & Saha, S. (2013). Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chemical Engineering Journal, 217, 426–434.
Panizza, M., Delucchi, M., & Cerisola, G. (2005). Electrochemical degradation of anionic surfactants. Journal of Applied Electrochemistry, 35(4), 357–361.
Parhizgar, F., Alishahi, A., Varasteh, H., & Rezaee, H. (2017). Removing sodium dodecyl benzene sulfonate (SDBS) from aqueous solutions using chitosan. Journal of Polymers and the Environment, 25(3), 836–843.
Pereira, L. C., de Souza, A. O., Bernardes, M. F. F., Pazin, M., Tasso, M. J., Pereira, P. H., & Dorta, D. J. (2015). A perspective on the potential risks of emerging contaminants to human and environmental health. Environmental Science and Pollution Research, 22(18), 13800–13823.
Pérez-Carrera, E., León, V. M., Lara-Martín, P. A., & González-Mazo, E. (2010). Influence of the hydrophilic moiety of anionic and nonionic surfactants on their aerobic biodegradation in seawater. Science of the Total Environment, 408(4), 922–930.
Runtti, H., Luukkonen, T., Niskanen, M., Tuomikoski, S., Kangas, T., Tynjälä, P., Tolonen, E.-T., Sarkkinen, M., Kemppainen, K., Rämö, J., & Lassi, U. (2016). Sulphate removal over barium-modified blast-furnace-slag geopolymer. Journal of Hazardous Materials, 317, 373–384.
Schouten, N., van der Ham, L. G., Euverink, G.-J. W., & de Haan, A. B. (2007). Selection and evaluation of adsorbents for the removal of anionic surfactants from laundry rinsing water. Water Research, 41(18), 4233–4241.
Shamsuddin, R. M., Verbeek, C. J. R., & Lay, M. C. (2014). Producing protein intercalated bentonite — equilibrium, kinetics and physical properties of gelatin–bentonite system. Applied Clay Science, 87, 52–60.
Sineva, A. V., Parfenova, A. M., & Fedorova, A. A. (2007). Adsorption of micelle forming and non-micelle forming surfactants on the adsorbents of different nature. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 306(1), 68–74.
Siyal, A. A., K. A. Azizli, L. Ismail, Z. Man and M. I. Khan (2016). Suitability of Malaysian fly ash for geopolymer synthesis. Advanced Materials Research, Trans Tech Publ.
Siyal, A. A., Rashid Shamsuddin, M., Rabat, N. E., Zulfiqar, M., Ayoub, M., & Azizli, K. A. (2018). Removal of anionic surfactant sodium dodecylbenzenesulfonate from water using fly ash adsorbent. IOP Conference Series: Materials Science and Engineering, 458, 012043.
Siyal, A. A., Shamsuddin, M. R., Rabat, N. E., Zulfiqar, M., Man, Z., & Low, A. (2019). Fly ash based geopolymer for the adsorption of anionic surfactant from aqueous solution. Journal of Cleaner Production, 229, 232–243.
Siyal, A. A., Shamsuddin, M. R., Low, A., & Rabat, N. E. (2020). A review on recent developments in the adsorption of surfactants from wastewater. Journal of Environmental Management, 254, 109797.
Taffarel, S. R., & Rubio, J. (2010). Adsorption of sodium dodecyl benzene sulfonate from aqueous solution using a modified natural zeolite with CTAB. Minerals Engineering, 23(10), 771–779.
Temkin, M. I. (1941). Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zh Fiz Chim, 15, 296–332.
Tezel, U., Tandukar, M., Martinez, R. J., Sobecky, P. A., & Pavlostathis, S. G. (2012). Aerobic biotransformation of n-tetradecylbenzyldimethylammonium chloride by an enriched pseudomonas spp. community. Environmental Science & Technology, 46(16), 8714–8722.
Valizadeh, S., Younesi, H., & Bahramifar, N. (2016). Highly mesoporous K2CO3 and KOH/activated carbon for SDBS removal from water samples: batch and fixed-bed column adsorption process. Environmental Nanotechnology, Monitoring & Management, 6, 1–13.
Visa, M., & Duta, A. (2013). TiO2/fly ash novel substrate for simultaneous removal of heavy metals and surfactants. Chemical Engineering Journal, 223, 860–868.
Wang, S., Boyjoo, Y., Choueib, A., & Zhu, Z. H. (2005). Removal of dyes from aqueous solution using fly ash and red mud. Water Research, 39(1), 129–138.
Wang, S., Terdkiatburana, T., & Tadé, M. O. (2008). Single and co-adsorption of heavy metals and humic acid on fly ash. Separation and Purification Technology, 58(3), 353–358.
Weber, W. J., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, 89(2), 31–60.
Zanoletti, A., Federici, S., Borgese, L., Bergese, P., Ferroni, M., Depero, L. E., & Bontempi, E. (2017). Embodied energy as key parameter for sustainable materials selection: the case of reusing coal fly ash for removing anionic surfactants. Journal of Cleaner Production, 141, 230–236.
Zhang, C., Valsaraj, K. T., Constant, W. D., & Roy, D. (1999). Aerobic biodegradation kinetics of four anionic and nonionic surfactants at sub-and supra-critical micelle concentrations (CMCs). Water Research, 33(1), 115–124.
Zhang, C., Wen, H., Huang, Y., & Shi, W. (2017). Adsorption of anionic surfactants from aqueous solution by high content of primary amino crosslinked chitosan microspheres. International Journal of Biological Macromolecules, 97, 635–641.
Zulfiqar, M., Chowdhury, S., Samsudin, M. F. R., Siyal, A. A., Omar, A. A., Ahmad, T., & Sufian, S. (2020). Effect of organic solvents on the growth of TiO2 nanotubes: an insight into photocatalytic degradation and adsorption studies. Journal of Water Process Engineering, 37, 101491.
Acknowledgments
Authors are thankful to Universiti Teknologi PETRONAS, Malaysia, for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siyal, A.A., Shamsuddin, R., Low, A. et al. Adsorption Kinetics, Isotherms, and Thermodynamics of Removal of Anionic Surfactant from Aqueous Solution Using Fly Ash. Water Air Soil Pollut 231, 509 (2020). https://doi.org/10.1007/s11270-020-04879-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04879-2