Abstract
The experiment was performed in support of a Japanese initiative to investigate the biological effects of irradiation from residual neutron-activated radioactivity that resulted from the A-bombing. Radionuclide 56Mn (T1/2 = 2.58 h) is one of the main neutron-activated emitters during the first hours after neutron activation of soil dust particles. In our previous studies (2016–2017) related to irradiation of male Wistar rats after dispersion of 56MnO2 powder, the internal doses in rats were found to be very inhomogeneous: distribution of doses among different organs ranged from 1.3 Gy in small intestine to less than 0.0015 Gy in some of the other organs. Internal doses in the lungs ranged from 0.03 to 0.1 Gy. The essential pathological changes were found in lung tissue of rats despite a low level of irradiation. In the present study, the dosimetry investigations were extended: internal doses in experimental mice and rats were estimated for various activity levels of dispersed neutron-activated 56MnO2 powder. The following findings were noted: (a) internal radiation doses in mice were several times higher in comparison with rats under similar conditions of exposure to 56MnO2 powder. (b) When 2.74 × 108 Bq of 56MnO2 powder was dispersed over mice, doses of internal irradiation ranged from 0.81 to 4.5 Gy in the gastrointestinal tract (small intestine, stomach, large intestine), from 0.096 to 0.14 Gy in lungs, and doses in skin and eyes ranged from 0.29 to 0.42 Gy and from 0.12 to 0.16 Gy, respectively. Internal radiation doses in other organs of mice were much lower. (c) Internal radiation doses were significantly lower in organs of rats with the same activity of exposure to 56MnO2 powder (2.74 × 108 Bq): 0.09, 0.17, 0.29, and 0.025 Gy in stomach, small intestine, large intestine, and lungs, respectively. (d) Doses of internal irradiation in organs of rats and mice were two to four times higher when they were exposed to 8.0 × 108 Bq of 56MnO2 (in comparison with exposure to 2.74 × 108 Bq of 56MnO2). (e) Internal radiation doses in organs of mice were 7–14 times lower with the lowest 56MnO2 amount (8.0 × 107 Bq) in comparison with the highest amount, 8.0 × 108 Bq, of dispersed 56MnO2 powder. The data obtained will be used for interpretation of biological effects in experimental mice and rats that result from dispersion of various levels of neutron-activated 56MnO2 powder, which is the subject of separate studies.
Similar content being viewed by others
Introduction
Our experiments were performed in support of a Japanese initiative to investigate the biological effects of irradiation from residual neutron-activated radioactivity that resulted from the A-bombing (Hoshi 2020). During nuclear explosions that take place in the atmosphere, neutron-activated radionuclides are distributed in surface layers of the soil, contributing to the beta and gamma irradiation that results from residual radioactivity. The main radionuclides are 24Na, 28Al, 31Si, 32P, 38Cl, 42K, 45Ca, 46Sc, 56Mn, 59Fe, 60Co, and 134Cs (Weitz 2014). Radionuclide 56Mn (T1/2 = 2.58 h) is one of the main neutron-activated emitters during the first hours after neutron activation of soil dust particles (Tanaka et al. 2008; Weitz 2014). The purpose of this international multicenter study was to extend our previous work (Shichijo et al. 2017; Stepanenko et al. 2017) to estimate internal doses for laboratory animals (mice and rats) with different exposures to 56MnO2 in the form of dispersed powder. The results of the internal dose assessments will be used to investigate the biological effects that result from this type of exposure, which will be the subject of future publications.
Materials and methods
Table 1 gives details of the laboratory mice and rats used in the experiments and also the initial 56Mn activity (100 mg 56MnO2 powder sprayed over the animals while they were in their cages).
The total numbers of mice and rats targeted for dosimetry only were 24 and 9, respectively. Along with the animals scheduled for dosimetry, animals that were intended for subsequent biological studies were additionally placed in the same cages. As a result, the total number of animals in each cage for each irradiation was different, from 6 to 9 rats and from 3 to 10 mice per cage.
All experimental work was performed during 2018–2019 at research reactor IVG.1 (“Baikal-1”) located in the territory of the Semipatinsk nuclear test site (Lanin 2013), Republic of Kazakhstan. Details of neutron activation of MnO2 powder (Rare Metallic Co., Ltd) and exposure of animals to dispersed 56MnO2 particles were presented in our previous paper (Stepanenko et al. 2017). Briefly, experimental animals were placed in special boxes for exposure to 56MnO2 powder (Fig. 1). One hundred milligrams of activated powder was used for each 56MnO2 exposure. Statistical distribution of MnO2 particle sizes is presented in Fig. 2. Animals were exposed for 1 h. Exposed animals were removed from cages and euthanized by injection of an excessive dose of pentobarbital. All work with experimental animals was approved by the ethics committee of Semey State Medical University, Kazakhstan, according to directive 2010/63/EU of the European Parliament and the Council of the Office on protection of animals used for scientific purposes of 22 September 2010 (Directive 2010/63/EU 2010). The following organs and tissues were surgically extracted from experimental animals: lungs, heart, small intestine, large intestine, stomach, esophagus, liver, spleen, kidney, trachea, skin, eyes, and blood. To measure specific activity of 56Mn, small pieces (about 1 ml) of each organ were weighed and subjected to gamma-spectrometry by an AMPTEC, Inc., Gamma-Rad5 spectrometer with an NaI(Tl) detector. Details of measurement conditions and calibration of the spectrometer were presented in our previous paper (Stepanenko et al. 2017). A description of internal dose estimations according to the Medical Internal Radiation Dose methodology (Bolch et al. 2009) was presented in the same paper. According to MIRD methodology, internal radiation doses were assessed by taking into account accumulated activity of 56Mn in all studied organs (which are listed above), self-irradiation of these organs, and their irradiation by all other sampled organs and tissues. Calculation of absorbed fractions of energy in studied organs from beta and gamma irradiation of 56Mn was performed using the Monte-Carlo method (Briemeister 2000) and age-dependent mathematical phantoms of rats and mice (Stepanenko et al. 2015). The spectrum of 56Mn beta particles (Stabin et al. 2001) was accounted for internal dose calculations. Gamma irradiation from 56Mn (Be et al. 2013) was accounted for as well.
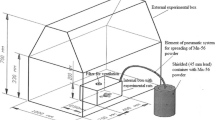
Schematic view of the box where neutron-activated radioactive 56MnO2 powder was dispersed on experimental animals. 1-Pneumatic tube for dispersion of radioactive 52Mn powder; 2-air filter; 3-plastic wall of the box; 4-plastic floor of the box with holes, where experimental animals were placed; 5-tubes for forced ventilation
Results
Each extracted sample of organs (lungs, heart, small intestine, large intestine, stomach, esophagus, liver, spleen, kidney, trachea, skin, eyes, and blood) from all investigated laboratory animals was subjected to gamma spectrometry in a well-shielded room. Volumes of extracted samples were small enough (about 1 ml) to consider them as radiating point sources in comparison with distance to and size of the spectrometer’s detector. The highest 56Mn specific activities were found in large and small intestine, stomach, lungs, and skin, which corresponds to our previous results obtained from similar experiments on rats (Stepanenko et al. 2017). A typical gamma spectra of 56Mn measured by a gamma-spectrometer are presented in Figs. 3 and 4. In both examples with measured gamma spectrum of 56Mn in biological samples, the amount of 56MnO2 powder dispersed over the experimental animals was equal. The background gamma spectrum measured in a well-shielded room inside the reactor building is presented in Fig. 5.
Examples of calculated specific absorbed fractions (SAF—absorbed fraction of emitted energy per unit of organ’s mass) for gammas and electrons as a function of energy are shown in Figs. 6, 7, 8, 9.
Accumulated doses of internal irradiation were estimated from the beginning of exposure until infinity. It was assumed that physical decay of 56Mn was essentially faster than biological redistribution of MnO2 powder in the experimental animals. Results of internal dose estimations are presented in Tables 2 and 3.
Discussion
In the present study, we found that under similar exposure conditions to 56MnO2 powder, the internal doses in mice were several times higher in comparison with rats. This can, perhaps, be explained by the following: higher breathing rate in mice versus rats and, lower organ weight in mice compared with rats (Besyadovsky et al. 1978). It should be noted that the latter circumstance leads to the fact that the specific absorbed fraction of energy (that is, fraction of absorbed energy per unit mass of the organ) is essentially higher in mice than in rats (see Figs. 6, 7, 8, 9). Difference in doses of internal irradiation of mice with the same activity of 56MnO2 powder dispersed over the experimental animals can be explained by the fact that the number of mice per cage was different during different irradiation sessions (see Table 3 with corresponding note). This can also explain the absence of a simple proportionality between the internal radiation doses and the dispersed activity of 56MnO2 (Tables 2 and 3). The increased doses in the lungs are explained by the fact that this organ is critical when inhaling small radioactive particles of 56MnO2, which leads to an increased accumulation of activity in this organ. High doses of irradiation of the gastrointestinal tract can be explained by the fact that in the process of cleaning and grooming, experimental animals swallowed radioactive particles retained by their hair, which led to a high accumulation of activity in the stomach and intestines during exposure (1 h), as it was noted in Stepanenko et al. (2017), Shichijo et al. (2017). The retention of radioactive particles by animal hair leads to an increase in skin radiation dose.
Conclusion
This study aimed to estimate internal doses in laboratory animals (mice and rats) that had been exposed to various levels of 56MnO2 in the form of dispersed powder. The experiment was performed in support of the Japanese initiative to investigate the biological effects of irradiation from residual neutron-activated radioactivity that resulted from the A-bombing (Hoshi 2020; Roesch 1987; Imanaka et al. 2012; Kerr et al. 2013, 2015; Ohtaki et al. 2014). Radionuclide 56Mn (T1/2 = 2.58 h) is one of the main neutron-activated emitters during the first hours after neutron activation of soil dust particles.
In our previous studies (Stepanenko et al. 2017; Shichijo et al. 2017) related to irradiation of male Wistar rats after dispersion of 56MnO2 powder, the internal doses in rats were found to be very inhomogeneous: distribution of doses among different organs ranged from 1.3 Gy in small intestine to less than 0.0015 Gy in some of the other organs. Internal doses in the lungs ranged from 0.03 to 0.1 Gy. The essential pathological changes were found in lung tissue of rats despite a low level of irradiation.
In the present study, the dosimetry investigations were extended: internal doses in experimental mice and rats were estimated for various activity levels of dispersed neutron-activated 56MnO2 powder.
The following findings were noted:
-
(a)
Internal radiation doses in mice were several times higher in comparison with rats under similar conditions of exposure to 56MnO2 powder.
-
(b)
When 2.74 × 108 Bq of 56MnO2 powder was dispersed over mice, doses of internal irradiation ranged from 0.81 to 4.5 Gy in the gastrointestinal tract (small intestine, stomach, large intestine), from 0.096 to 0.14 Gy in lungs, and doses in skin and eyes ranged from 0.29 to 0.42 Gy and from 0.12 to 0.16 Gy, respectively. Internal radiation doses in other organs of mice were much lower.
-
(c)
Internal radiation doses were significantly lower in organs of rats with the same activity of exposure to 56MnO2 powder (2.74 × 108 Bq): 0.09, 0.17, 0.29, and 0.025 Gy in stomach, small intestine, large intestine, and lungs, respectively.
-
(d)
Doses of internal irradiation in organs of rats and mice were two to four times higher when they were exposed to 8.0 × 108 Bq of 56MnO2 (in comparison with exposure to 2.74 × 108 Bq of 56MnO2).
-
(e)
Internal radiation doses in organs of mice were 7–14 times lower with the lowest 56MnO2 amount (8.0 × 107 Bq) in comparison with the highest amount, 8.0 × 108 Bq, of dispersed 56MnO2 powder.
The data obtained will be used for interpretation of biological effects in experimental mice and rats that result from dispersion of various levels of neutron-activated 56MnO2 powder, which is the subject of separate studies.
References
Be M-M, Chiste V, Dulieu C, Mougeot X, Browne E, Baglin C, Chechnev VP, Egorov A, Kuzmenko NK, Sergeev VO, Kondev FG, Luca A, Galan M, Huang X, Wang B, Helmer RG, Schonfeld E, Dersch R, Vanin VR, de Castro RM, Nichols AL, MacMahon TD, Pearce A, Arinc A, Lee KB, Wu SC (2013) Table of radionuclides (Comments on evaluation). Volumes 1–7, Bureau International des Poids et Mesures. Paviollon de Breteuil, F-92310 SEVRES. https://www.bipm.org/utils/common/pdf/monographieRI/Monographie_BIPM-5_Tables_Vol7.pdf .Accessed 20 Feb 2020
Besyadovsky RA, Ivanov KV, Kozyura AK (1978) Handbook for the radiobiologists. Moscow. Atomizdat. pp. 78 (in Russian). https://www.ozon.ru/context/detail/id/17918088/. Accessed 20 Feb 2020
Bolch WE, Eckerman KF, Sgouros G, Thomas R (2009) MIRD Pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry-standardization of nomenclature. J Nucl Med 50(3):477–484. https://doi.org/10.2967/jnumed.108.056036. http://www.hopkinsmedicine.org/RTD_lab/pdf/2009_JNM_MIRD_P21_schema.pdf. Accessed 20 Feb 2020
Briemeister JF (2000) MCNP–A general Monte–Carlo N–particle transport code. In: Version 4C. Los Alamos. Los Alamos National Laboratory, USA
Directive 2010/63/EU of the European Parliament and the Council of the Office on the protection of animals used for scientific purposes of 22 September 2010 (2010) Official Journal of the European Union. L 276: 33–79. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:EN:PDF. Accessed 20 February 2020
Hoshi M (2020) A long history exploring radiation exposure. Impact, pp 70–72. https://www.ingentaconnect.com/content/sil/impact/2020/00002020/00000003/art00026?crawler=true&mimetype=application/pdf
Imanaka T, Endo S, Kawano N, Tanaka K (2012) Radiation exposure and disease questionnaires of early entrants after the Hiroshima bombing. Radiat Protect Dosim 149(1): 91–96. https://doi.org/10.1093/rpd/ncr370. https://www.ncbi.nlm.nih.gov/pubmed/21914640. Accessed 20 Feb 2020
Kerr GD, Egbert SD, Al-Nabulsi I, Beck HL, Cullings HM, Endo S, Hoshi M, Imanaka T, Kaul DC, Maruyama S, Reeves GI, Ruehm W, Sakaguchi A, Simon SL, Spriggs GD, Stram DO, Tonda T, Weiss JF, Weitz RL, Young RW (2013) Workshop report on atomic bomb dosimetry—residual radiation exposure: recent research and suggestions for future studies. Health Phys 105(2):140–149. https://doi.org/10.1097/hp.0b013e31828ca73a. http://energy.gov/ehss/downloads/workshop-report-health-physics-journal. Accessed 20 Feb 2020
Kerr GD, Egbert SD, Al-Nabulsi I, Bailiff IK, Beck HL, Belukha IG, Cockayne JE, Cullings HM, Eckerman KF, Granovskaya E, Grant EJ, Hoshi M, Kaul DC, Kryuchkov V, Mannis D, Ohtaki M, Otani K, Shinkarev S, Simon SL, Spriggs GD, Stepanenko VF, Stricklin D, Weiss JF, Weitz RL, Woda C, Worthington PR, Yamamoto K, Young RW (2015) Workshop report on atomic bomb dosimetry—a review of dose related factors for the evaluation of exposures to residual radiation at Hiroshima and Nagasaki. Health Phys 109(6):582–600. https://doi.org/10.1097/hp.0000000000000395. https://www.ncbi.nlm.nih.gov/pubmed/26509626. Accessed 20 Feb 2020
Lanin A (2013) Nuclear rocket engine reactor. Springer Ser Mater Sci 170:1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32430-7_1. https://www.springer.com/gp/book/9783642324291. Accessed 20 Feb 2020
Ohtaki M, Otani K, Tonda T, Sato Y, Hara N, Imori S, Matsui C, Kawakami H, Tashiro S, Aihara K, Hoshi M, Satoh K (2014) Effect of distance from hypocenter at exposure on solid cancer mortality among Hiroshima atomic bomb survivors with very low initial radiation dose in the Dosimetry System 1986 (DS86). Health Phys 107(1):S45
Roesch WC (eds) (1987) US—Japan Joint Reassessment of Atomic Bomb Radiation Dosimetry in Hiroshima and Nagasaki, Final Report-Dosimetry System 1986 (DS86). Radiation Effects Research Foundation. Hiroshima. http://www.rerf.jp/library/index_e.html. Accessed 20 Feb 2020
Shichijo K, Fujimoto N, Uzbekov D, Kairkhanova Y, Saimova A, Chaizhunusova N, Sayakenov N, Shabdarbaeva D, Aukenov N, Azimkhanov A, Kolbayenkov A, Mussazhanova Z, Niino D, Nakashima M, Zhumadilov K, Stepanenko V, Tomonaga M, Rakhypbekov T, Hoshi M (2017) Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats—part 2: pathological effects. Radiat Environ Biophys 56(1):55–61. https://doi.org/10.1007/s00411-016-0676-z. https://link.springer.com/article/10.1007/s00411-016-0676-z. Accessed February 20, 2020
Stabin M, Hunt SJ, Sparks R, Lipsztein J, Eckerman J (2001) RADAR: the radiation dose assessment resource–an online source of dose information for nuclear medicine and occupation radiation safety. J Nucl Med 42:243
Stepanenko VF, Iaskova EK, Belukha IG, Petriev VM, Skvortsov VG, Kolyzhenkov TV, Petukhov AD, Dubov DV (2015) The calculation of internal irradiation of nano-, micro- and macro-biostructures by electrons, beta particles and quanta radiation of different energy for the development and research of new radiopharmaceuticals in nuclear medicine. Radiation and risk. Bull Natl Radiat Epidemiol Registry 24(1):35–60
Stepanenko V, Rakhypbekov T, Otani K, Endo S, Satoh K, Kawano N, Shichijo K, Nakashima M, Takatsuji T, Sakaguchi A, Kato H, Onda Y, Fujimoto N, Toyoda S, Sato H, Dyussupov A, Chaizhu-nusova N, Sayakenov N, Uzbekov D, Saimova A, Shabdarbaeva D, Skakov M, Vurim A, Gnyrya V, Azimkhanov A, Kolbayenkov A, Zhumadilov K, Kairikhanova Y, Kaprin A, Galkin V, Ivanov S, Kolyzhenkov T, Petukhov A, Yaskova E, Belukha I, Khailov A, Skvortsov V, Ivannikov A, Akhmedova U, Bogacheva V, Hoshi M (2017) Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats—part 1: dosimetry. Radiat Environ Biophys 56: 47–54. https://doi.org/10.1007/s00411-016-0678-x. https://www.ncbi.nlm.nih.gov/pubmed/28188481. Accessed 20 Feb 2020
Tanaka K, Endo S, Imanaka T, Shizuma K, Hasai H, Hoshi M (2008) Skin dose from neutron-activated soil for early entrants following the A-bomb detonation in Hiroshima: contribution from beta and gamma rays. Radiat Environ Biophys 47:323–330. https://doi.org/10.1007/s00411-008-0172-1. https://www.ncbi.nlm.nih.gov/pubmed/18496704. Accessed 20 Feb 2020
Weitz R (2014) Reconstruction of beta-particle and gamma-ray doses from neutron activated soil at Hiroshima and Nagasaki. Health Phys 107(1):S43
Acknowledgements
In Japan, this research was supported by JSPS KAKENHI Grants nos. 26257501 (April 2014–March 2018), 19H01149 (April 2019–March 2023), and 19KK0266, Japan. In Kazakhstan, this research was supported by Semey State Medical University, Republic of Kazakhstan. A. Tsyb Medical Radiological Research Center–National Medical Research Center of Radiology, Ministry of Health of Russian Federation supported the research by providing gamma spectrometry and internal dose estimations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors of this paper have no conflicts of interest according to their disclosure forms.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stepanenko, V., Kaprin, A., Ivanov, S. et al. Internal doses in experimental mice and rats following exposure to neutron-activated 56MnO2 powder: results of an international, multicenter study. Radiat Environ Biophys 59, 683–692 (2020). https://doi.org/10.1007/s00411-020-00870-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-020-00870-x