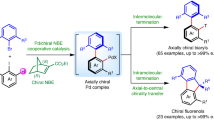
Abstract
Carbocations can be categorized into classical carbenium ions and non-classical carbonium ions. These intermediates are ubiquitous in reactions of both fundamental and practical relevance, finding application in the petroleum industry as well as the discovery of new drugs and materials. Conveying stereochemical information to carbocations is therefore of interest to a range of chemical fields. While previous studies targeted systems proceeding through classical ions, enantiocontrol over their non-classical counterparts has remained unprecedented. Here we show that strong and confined chiral acids catalyse enantioselective reactions via the non-classical 2-norbornyl cation. This reactive intermediate is generated from structurally different precursors by leveraging the reactivity of various functional groups to ultimately deliver the same enantioenriched product. Our work demonstrates that tailored catalysts can act as suitable hosts for simple, non-functionalized carbocations via a network of non-covalent interactions. We anticipate that the methods described herein will provide catalytic accessibility to valuable carbocation systems.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The experimental procedures and analytical data supporting the findings of this study are available within the manuscript and its Supplementary Information file. Raw and unprocessed NMR data are available from the corresponding author upon reasonable request. Crystallographic data for compounds 3 (CCDC 1948386) and 7 (crystal 1, CCDC 2021275; crystal 2, CCDC 1948044; crystal 3, CCDC 1948043; crystal 4, CCDC 2021276) can be downloaded free of charge from the Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk).
References
Weininger, S. J. “What’s in a name?” From designation to denunciation—the nonclassical cation controversy. Bull. Hist. Chem. 25, 123–131 (2000).
Brown, H. C. The Nonclassical Ion Problem 1st edn (Plenum Press, 1977).
Schleyer, P. v. R., Mainz, V. V. & Strom, E. T. in The Foundations of Physical Organic Chemistry: Fifty Years of the James Flack Norris Award Vol. 1209 ACS Symposium Series Ch. 7, 139–168 (American Chemical Society, 2015).
Scholz, F. et al. Crystal structure determination of the nonclassical 2-norbornyl cation. Science 341, 62–64 (2013).
Olah, G. A. 100 years of carbocations and their significance in chemistry. J. Org. Chem. 66, 5943–5957 (2001).
Olah, G. A. Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- of tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions. J. Am. Chem. Soc. 94, 808–820 (1972).
Olah, G. A. My search for carbocations and their role in chemistry (Nobel lecture). Angew. Chem. Int. Ed. 34, 1393–1405 (1995).
Naredla, R. R. & Klumpp, D. A. Contemporary carbocation chemistry: applications in organic synthesis. Chem. Rev. 113, 6905–6948 (2013).
Isomura, M., Petrone, D. A. & Carreira, E. M. Coordination-induced stereocontrol over carbocations: asymmetric reductive deoxygenation of racemic tertiary alcohols. J. Am. Chem. Soc. 141, 4738–4748 (2019).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Zhao, C., Toste, F. D., Raymond, K. N. & Bergman, R. G. Nucleophilic substitution catalyzed by a supramolecular cavity proceeds with retention of absolute stereochemistry. J. Am. Chem. Soc. 136, 14409–14412 (2014).
Braun, M. & Kotter, W. Titanium(iv)-catalyzed dynamic kinetic asymmetric transformation of alcohols, silyl ethers, and acetals under carbon allylation. Angew. Chem. Int. Ed. 43, 514–517 (2004).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. 52, 534–561 (2013).
Tsuji, N. et al. Activation of olefins via asymmetric Brønsted acid catalysis. Science 359, 1501–1505 (2018).
Brown, H. C., Prasad, J. V. N. V. & Zaidlewicz, M. Hydroboration. 83. Asymmetric hydroboration of representative cis disubstituted and heterocyclic olefins with dicaranylboranes of high enantiomeric purity. J. Org. Chem. 53, 2911–2916 (1988).
Burgess, K. & Ohlmeyer, M. J. Enantioselective hydroboration mediated by homochiral rhodium catalysts. J. Org. Chem. 53, 5178–5179 (1988).
Dorta, R., Egli, P., Zürcher, F. & Togni, A. The [IrCl(diphosphine)]2/fluoride system. Developing catalytic asymmetric olefin hydroamination. J. Am. Chem. Soc. 119, 10857–10858 (1997).
Sevov, C. S., Zhou, J. & Hartwig, J. F. Iridium-catalyzed intermolecular hydroamination of unactivated aliphatic alkenes with amides and sulfonamides. J. Am. Chem. Soc. 134, 11960–11963 (2012).
Gountchev, T. I. & Tilley, T. D. Hydrosilylation catalysis by C2-symmetric bis(silylamido) complexes of yttrium. Organometallics 18, 5661–5667 (1999).
Eichberger, G., Penn, G., Faber, K. & Griengl, H. Large scale preparation of (+)- and (–)- endo-norborneol by enzymatic hydrolysis. Tetrahedron Lett. 27, 2843–2844 (1986).
Ma, L., Sweet, E. H. & Schultz, P. G. Selective antibody-catalyzed solvolysis of endo-2-norbornyl mesylate. J. Am. Chem. Soc. 121, 10227–10228 (1999).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition, and applications. Angew. Chem. Int. Ed. 52, 518–533 (2013).
Hong, Y. J. & Tantillo, D. J. Perturbing the structure of the 2-norbornyl cation through C–H···N and C–H··· π interactions. J. Org. Chem. 72, 8877–8881 (2007).
Hong, Y. J. & Tantillo, D. J. C–H⋯ π interactions as modulators of carbocation structure—implications for terpene biosynthesis. Chem. Sci. 4, 2512–2518 (2013).
Wagner, J. P. & Schreiner, P. R. London dispersion in molecular chemistry—reconsidering steric effects. Angew. Chem. Int. Ed. 54, 12274–12296 (2015).
Winstein, S. & Trifan, D. S. The structure of the bicyclo[2,2,1]2-heptyl (norbornyl) carbonium ion. J. Am. Chem. Soc. 71, 2953–2953 (1949).
Overman, L. E. Thermal and mercuric ion catalyzed [3,3]-sigmatropic rearrangement of allylic trichloroacetimidates. The 1,3 transposition of alcohol and amine functions. J. Am. Chem. Soc. 96, 597–599 (1974).
Akiyama, T. & Mori, K. Stronger Brønsted acids: recent progress. Chem. Rev. 115, 9277–9306 (2015).
James, T., van Gemmeren, M. & List, B. Development and applications of disulfonimides in enantioselective organocatalysis. Chem. Rev. 115, 9388–9409 (2015).
Čorić, I. & List, B. Asymmetric spiroacetalization catalysed by confined Brønsted acids. Nature 483, 315–319 (2012).
Liu, L., Kaib, P. S. J., Tap, A. & List, B. A general catalytic asymmetric Prins cyclization. J. Am. Chem. Soc. 138, 10822–10825 (2016).
Fabbri, D., Delogu, G. & de Lucchi, O. Thiophosphonates of 1,1-binaphthol as chiral equivalents of H2S. Preparation of 2-mercaptonorbornanes and 2-mercaptonorbornenes. Tetrahedron Asymmetry 4, 1591–1596 (1993).
Kaib, P. S. J., Schreyer, L., Lee, S., Properzi, R. & List, B. Extremely active organocatalysts enable a highly enantioselective addition of allyltrimethylsilane to aldehydes. Angew. Chem. Int. Ed. 55, 13200–13203 (2016).
Schreyer, L., Properzi, R. & List, B. IDPi catalysis. Angew. Chem. Int. Ed. 58, 12761–12777 (2019).
Winstein, S. & Trifan, D. Neighboring carbon and hydrogen. XI. Solvolysis of exo-norbornyl p-bromobenzenesulfonate. J. Am. Chem. Soc. 74, 1154–1160 (1952).
Winstein, S. & Trifan, D. Neighboring carbon and hydrogen. X. Solvolysis of endo-norbornyl arylsulfonates. J. Am. Chem. Soc. 74, 1147–1154 (1952).
Olah, G. A., White, A. M., DeMember, J. R., Commeyras, A. & Lui, C. Y. Stable carbonium ions. C. Structure of the norbornyl cation. J. Am. Chem. Soc. 92, 4627–4640 (1970).
Amii, H. & Uneyama, K. C–F bond activation in organic synthesis. Chem. Rev. 109, 2119–2183 (2009).
Jaiswal, A. K., Prasad, P. K. & Young, R. D. Nucleophilic substitution of aliphatic fluorides via pseudohalide intermediates. Chem. Eur. J. 25, 6290–6294 (2019).
Lawton, R. G. 1,5 participation in the solvolysis of β-(Δ3-cyclopentenyl)-ethyl p-nitrobenzenesulfonate. J. Am. Chem. Soc. 83, 2399–2399 (1961).
Saunders, M., Schleyer, P. v. R. & Olah, G. A. Stable carbonium ions. XI. The rate of hydride shifts in the 2-norbornyl cation. J. Am. Chem. Soc. 86, 5680–5681 (1964).
Olah, G. A., Prakash, G. K. S. & Saunders, M. Conclusion of the classical-nonclassical ion controversy based on the structural study of the 2-norbornyl cation. Acc. Chem. Res. 16, 440–448 (1983).
Tantillo, D. J. The carbocation continuum in terpene biosynthesis—where are the secondary cations? Chem. Soc. Rev. 39, 2847–2854 (2010).
Acknowledgements
Support from the Max Planck Society, the Deutsche Forschungsgemeinschaft (Leibniz Award to B.L. and Cluster of Excellence Ruhr Explores Solvation (RESOLV, EXC 1069)), the European Research Council (Advanced Grant ‘C–H Acids for Organic Synthesis, CHAOS’), the Alexander-von-Humboldt Foundation (fellowship to L.S.), and the Fonds der Chemischen Industrie (fellowship to G.P.) is acknowledged. We thank the technicians of our group and all members of the service departments of the Max-Planck-Institut für Kohlenforschung, with a special mention to R. Goddard, N. Nöthling, A. Deege and H. Hinrichs. We thank J. L. Kennemur and L. Schreyer for discussions during the preparation of the manuscript. We are grateful to B. Mitschke for graphical suggestions and all group members that participated in the crowd reviewing process. We acknowledge A. Blond, D. Petkova and M. R. Monaco for their contributions to initial studies.
Author information
Authors and Affiliations
Contributions
B.L. and P.R.S. jointly developed the idea of this project; B.L. conceived, directed and oversaw the project; R.P. designed and conducted the experiments with the help of P.S.J.K.; R.P. and M.L. conducted the mechanistic investigations; M.L. performed the spectroscopic experiments and data analysis; G.P. initiated the experimental work and performed early reactivity studies; R.M. and C.K.D. first synthesized IDPi 3; L.S. carried out the computations; and R.P. and B.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.L., P.S.J.K. and R.P. are inventors on patent WO2017037141 (A1) filed by the Max-Planck-Institut für Kohlenforschung covering the IDPi catalyst class and its applications in asymmetric synthesis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, data, text, computational methods, Figs. 1–34 and Tables 1–16.
Supplementary Data 1
Crystallographic data for compound 7, crystal 1. CCDC reference 2021275.
Supplementary Data 2
Crystallographic data for compound 7, crystal 2. CCDC reference 1948044.
Supplementary Data 3
Crystallographic data for compound 7, crystal 3. CCDC reference 1948043.
Supplementary Data 4
Crystallographic data for compound 7, crystal 4. CCDC reference 2021276.
Supplementary Data 5
Crystallographic data for compound 3. CCDC reference 1948386.
Rights and permissions
About this article
Cite this article
Properzi, R., Kaib, P.S.J., Leutzsch, M. et al. Catalytic enantiocontrol over a non-classical carbocation. Nat. Chem. 12, 1174–1179 (2020). https://doi.org/10.1038/s41557-020-00558-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00558-1
This article is cited by
-
Catalytic asymmetric cationic shifts of aliphatic hydrocarbons
Nature (2024)
-
Enantioselective synthesis of tetraarylmethanes through meta-hydroxyl-directed benzylic substitution
Nature Synthesis (2023)
-
Enantioselective synthesis of amino acids from ammonia
Nature Catalysis (2022)