Abstract
Mitochondria contain the genetic information and expression machinery to produce essential respiratory chain proteins. Within the mitochondrial matrix, newly synthesized RNA, RNA processing proteins and mitoribosome assembly factors form punctate sub-compartments referred to as mitochondrial RNA granules (MRGs)1,2,3. Despite their proposed importance in regulating gene expression, the structural and dynamic properties of MRGs remain largely unknown. We investigated the internal architecture of MRGs using fluorescence super-resolution localization microscopy and correlative electron microscopy, and found that the MRG ultrastructure consists of compacted RNA embedded within a protein cloud. Using live-cell super-resolution structured illumination microscopy and fluorescence recovery after photobleaching, we reveal that MRGs rapidly exchange components and can undergo fusion, characteristic properties of fluid condensates4. Furthermore, MRGs associate with the inner mitochondrial membrane and their fusion coincides with mitochondrial remodelling. Inhibition of mitochondrial fission or fusion leads to an aberrant accumulation of MRGs into concentrated pockets, where they remain as distinct individual units despite their close apposition. Together, our findings reveal that MRGs are nanoscale fluid compartments, which are dispersed along mitochondria via membrane dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All imaging as well as numerical data relevant to this study are publicly available in the online repository Zenodo (https://doi.org/10.5281/zenodo.3747143) or upon reasonable request. A README-file on Zenodo will guide the reader. All remaining other data supporting the findings of this study are available from the corresponding author on reasonable request. Plasmids and cell lines are available; please contact the corresponding authors. Source data are provided with this paper.
Code availability
All code including adapted STORM-analysis code, TrackFRAP, FRAPtA and other python scripts and Fiji macros for analysis and figure generation are available in the online repository GitHub (https://github.com/TimoHenry/MitochondrialRNAgranules), or upon reasonable request. Jupyter Notebooks are available in the data repository on Zenodo to trace the application of the code to the data in our manuscript.
References
Antonicka, H., Sasarman, F., Nishimura, T., Paupe, V. & Shoubridge, E. A. The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 17, 386–398 (2013).
Iborra, F. J., Kimura, H. & Cook, P. R. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2, 9 (2004).
Jourdain, A. A. et al. GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metab. 17, 399–410 (2013).
Hyman, A. A., Weber, C. A. & Jülicher, F. Liquid–liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014).
Handwerger, K. E., Cordero, J. A. & Gall, J. G. Cajal bodies, nucleoli and speckles in the Xenopus oocyte nucleus have a low-density, sponge-like structure. Mol. Biol. Cell 16, 202–211 (2005).
Yamazaki, T. et al. Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. Mol. Cell 70, 1038–1053 (2018).
Feric, M. et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 (2016).
Frottin, F. et al. The nucleolus functions as a phase-separated protein quality control compartment. Science 365, 342–347 (2019).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Boeynaems, S. et al. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28, 420–435 (2018).
Langdon, E. M. et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 360, 922–927 (2018).
Maharana, S. et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 360, 918–921 (2018).
Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699 (2018).
Alberti, S., Gladfelter, A. & Mittag, T. Considerations and challenges in studying liquid–liquid phase separation and biomolecular condensates. Cell 176, 419–434 (2019).
McSwiggen, D. T. et al. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation.eLife 8, e47098 (2019).
Jourdain, A. A. et al. A mitochondria-specific isoform of FASTK is present in mitochondrial RNA granules and regulates gene expression and function. Cell Rep. 10, 1110–1121 (2015).
Jajoo, R. et al. Accurate concentration control of mitochondria and nucleoids. Science 351, 169–172 (2016).
Lewis, S. C., Uchiyama, L. F. & Nunnari, J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 353, aaf5549 (2016).
Douglass, K. M., Sieben, C., Archetti, A., Lambert, A. & Manley, S. Super-resolution imaging of multiple cells by optimized flat-field epi-illumination. Nat. Photon. 10, 705–708 (2016).
Alán, L., Špaček, T. & Ježek, P. Delaunay algorithm and principal component analysis for 3D visualization of mitochondrial DNA nucleoids by Biplane FPALM/dSTORM. Eur. Biophys. J. 45, 443–461 (2016).
Brown, T. A. et al. Superresolution fluorescence imaging of mitochondrial nucleoids reveals their spatial range, limits and membrane interaction. Mol. Cell. Biol. 31, 4994–5010 (2011).
Kukat, C. et al. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc. Natl Acad. Sci. USA 108, 13534–13539 (2011).
Ghezzi, D. et al. FASTKD2 nonsense mutation in an infantile mitochondrial encephalomyopathy associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 83, 415–423 (2008).
Yoo, D. H. et al. Identification of FASTKD2 compound heterozygous mutations as the underlying cause of autosomal recessive MELAS-like syndrome. Mitochondrion 35, 54–58 (2017).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Tu, Y. T. & Barrientos, A. The human mitochondrial DEAD-Box protein DDX28 resides in RNA granules and functions in mitoribosome assembly. Cell Rep. 10, 854–864 (2015).
Zaganelli, S. et al. The pseudouridine synthase RPUSD4 is an essential component of mitochondrial RNA granules. J. Biol. Chem. 292, 4519–4532 (2017).
Farge, G. et al. The N-terminal domain of TWINKLE contributes to single-stranded DNA binding and DNA helicase activities. Nucleic Acids Res. 36, 393–403 (2008).
Wheeler, J. R., Matheny, T., Jain, S., Abrisch, R. & Parker, R. Distinct stages in stress granule assembly and disassembly. eLife 5, e18413 (2016).
Garrido, N. et al. Composition and dynamics of human mitochondrial nucleoids. Mol. Biol. Cell 14, 1583–1596 (2003).
Stephan, T., Roesch, A., Riedel, D. & Jakobs, S. Live-cell STED nanoscopy of mitochondrial cristae. Sci. Rep. 9, 12419 (2019).
Souquere, S. et al. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. J. Cell Sci. 122, 3619–3626 (2009).
Gerhold, J. M. et al. Human mitochondrial DNA–protein complexes attach to a cholesterol-rich membrane structure. Sci. Rep. 5, 15292 (2015).
Hytti, M. et al. Antimycin A-induced mitochondrial damage causes human RPE cell death despite activation of autophagy.Oxid. Med. Cell. Longev. 2019, 1583656 (2019).
Ban-Ishihara, R., Ishihara, T., Sasaki, N., Mihara, K. & Ishihara, N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc. Natl Acad. Sci. USA 110, 11863–11868 (2013).
Jain, S. et al. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498 (2016).
Durigon, R. et al. LETM1 couples mitochondrial DNA metabolism and nutrient preference.EMBO Mol. Med. 10, e8550 (2018).
Ester, M., Kriegel, H.-P., Sander, J. & Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the 2nd International Conference on Knowledge Discovery and Data Mining 226–231 (AAAI, 1996).
Sieben, C., Banterle, N., Douglass, K. M., Gonczy, P. & Manley, S. Multicolor single-particle reconstruction of protein complexes. Nat. Methods 15, 777–780 (2018).
Spruyt, V. A Geometric Interpretation of the Covariance Matrix (Computer Vision for Dummies, 2014); https://www.visiondummy.com/2014/04/geometric-interpretation-covariance-matrix/
Ducret, A., Quardokus, E. M. & Brun, Y. V. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat. Microbiol 1, 16077 (2016).
Halavatyi, A., Yotskou, M. & Friederich, E. FRAPAnalyser (OMICtools, 2008); https://omictools.com/frapanalyser-tool
Weber, M. ‘statannot’ (GitHub, 2019); https://github.com/webermarcolivier/statannot
Acknowledgements
We thank H. Perreten for molecular cloning, F. Prodon for help with STED microscopy, O. Burri for the initial FRAPtrack code and the BIOP (EPFL) for imaging support. We thank R. Jajoo for his generous sharing of original code and data, and T. Stephan and S. Jakobs for the unreserved provision of Cox8a-SNAP cell lines and plasmids. We also thank M. Colomer and M. Martinez for their contribution to assessing MRG distribution. We are grateful to C. Sieben, K. Douglass, T. Kleele, J. Griffié and all members of the Manley and Martinou groups for discussions. Flow cytometry cell sorting was performed at the EPFL Flow Cytometry Core Facility. Electron microscopy was performed at the EPFL BioEM facility. Fluorescence microscopy was partially performed at the EPFL Bio Optics Platform (BIOP) and the UniGe Bio Imaging Core facility. This work was supported by the European Research Council (ERC CoG 819823, Piko to S.M. and T.R.) and the Swiss National Science Foundation (31003A_179421 to J.-C.M.).
Author information
Authors and Affiliations
Contributions
S.Z., T.R., J.-C.M. and S.M. conceived and designed the study and wrote the manuscript. All authors reviewed and edited the manuscript. T.R. and S.Z. designed, executed, analysed and validated the experiments. E.C. executed and coded FRAP experiments and analysis. E.V. performed fractionation and western blotting. M.C. embedded, sectioned and acquired transmission electron microscopy samples. T.R. and S.Z. prepared the figures and plots. S.M. and J.-C.M. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Workflow and quantification of nanoscopic architecture of MRGs.
a, Workflow used for this study. Previously unpublished parts of the analysis are highlighted by asterisks, while other parts were previously published17,36. b–f Additional quantification of MRG and nucleoid (mtDNA) architecture from htSTORM data. Markers (mtRNA, FASTKD2 and GRSF1), number of granules n and median values are indicated for each condition; n.s. denotes p-values > 0.05, * denotes p-values ≤ 0.05, ** denote p-values ≤ 0.01, *** denote p-values ≤ 0.001,**** denote p-values ≤ 0.0001 of two-sided Mann-Whitney-U test. Individual data points are plotted grey, box plots denote first and third quartiles, and the median, whiskers comprise rest of distributions except outliers. Multiple acquisitions, samples and imaging days were pooled. b, Median eccentricity of both MRG-proteins differ slightly (p = 5.1e−3), with largely overlapping boxes and can be approximated by spheres. Nucleoids and nascent RNA components of MRGs are more elongated (pBrU-FASTKD2 = 1.4e−15, pBrU-GRSF1 = 1.7e−6). c, Comparison of areas described by convex hull. MRG-protein foci are significantly larger than nascent-RNA foci (pBrU-FASTKD2 = 2.7 × 10-61, pBrU-GRSF1 = 6.5 × 10-49), yet less different from one another (pGRSF1-FASTKD2 = 6.9 × 10-7). The distribution of mtRNA- and mtDNA-foci areas strongly overlap, though their medians are significantly different with pmtRNA-mtDNA = 1.2 × 10-6. Three outliers for FASTKD2 (> 2.5 µm2) were removed for better visualisation, but included in all quantitative analysis. d, and e, Comparison of alternative standard point-cloud descriptors Radius of gyration (Rg), and Sigma as the average of the eigenvalues in two dimensions, and multiplied by two to yield a diameter. f, Density of localisations was also compared, and both GRSF1 and FASTKD2-foci follow a narrow normal distribution, while mtDNA & BrU show a larger variance of density. Number (n) of clusters quantified for each condition is represented in the figure and is pooled from 24, 13, 7, and 14 FOVs, and 4, 4, 3, and 2 samples for BrU, GRSF1, FASTKD2, and mtDNA respectively. Statistical source data are provided in Source data Extended data Fig. 1.
Extended Data Fig. 2 Comparison and correlation of two-colour htSTORM data.
a, Nine additional examples of two-colour htSTORM of MRGs. Scatter plots of localisations (right) are shown next to corresponding clusters of FASTKD2 (green) and mtRNA (BrU, blue) overlaid on widefield images (left). Convex hull areas are represented with dashed lines. b, and c, Scatter plots of all FASTKD2-mtRNA (BrU) pairs with regression-fit (black) and standard deviation (grey). Histograms of the distribution for FASTKD2 (y-axis, right, green), and mtRNA (x-axis, top, blue), including a kernel density estimate are shown. No correlation of Diameter (FWHM) (R = 0.26) or eccentricity (length/width, R = 0.43) was found between FASTKD2 and BrU foci from individual granules (n=26 MRGs over 4 independent experiments). d-f, Comparison of foci characteristics for one- versus two-colour htSTORM by Two-sided Mann-Whitney-U test from two-colour to one-colour data. Number of granules n (pooled from 20 FOVs, and 8 samples) and median values are indicated for each condition; n.s. denotes p-values > 0.05, * denotes p-values ≤ 0.05, ** denote p-values ≤ 0.01, *** denote p-values ≤ 0.001,**** denote p-values ≤ 0.0001. d, FWHM is not significant for FASTKD2 (p = 0.22) but two-colour BrU foci were significantly larger (p = 8.8e-4), and two-colour FASTKD2 were also larger than two-colour-mtRNA (p = 0.0014) e, Eccentricity is not significantly different (pmtRNA = 0.36, pFASTKD2 = 1.0) f, Size determined by convex hull, differed between one-colour and two-colour BrU (p = 2.4e-4) as well as FASTKD2 (p = 0.017). This may in parts be due to the heavy weight of two outliers as visible in the plot. Statistical source data are provided in Source data Extended data Fig. 2.
Extended Data Fig. 3 FRAP of MRG-associated proteins.
a, Representative time-lapse images of MRGs FRAP experiments in U2OS cells stably expressing FASTKD2-eGFP (green) (n= 39 MRGs examined over 3 independent experiments). White arrowheads indicate the photobleached structures. Scale bar: 5 μm. b, FRAP analysis of FASTKD2-eGFP in U2OS (n= 39 MRGs examined over 3 independent experiments) and COS-7 cells (n = 44 MRGs examined over 8 independent experiments). Symbols in the graph represent mean data points. Single exponential fits (lines) and standard deviations for each time point (shaded area) are shown. c, d, Representative time-lapse images of ERAL1- (n = 17 MRGs examined over 3 independent experiments) and DDX28-eGFP (n = 17 MRGs examined over 3 independent experiments) FRAP experiments in COS-7 cells. White arrowheads indicate the photobleached structures. These images correspond to the data plotted in Fig. 2c. Scale bar: 2 µm.
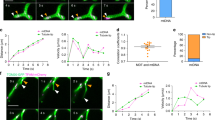
Extended Data Fig. 4 MRG and nucleoid dynamics in live cells.
a, Representative time-lapse images of an MRG fusion event in a live U2OS cell, monitored by SIM. MRGs are visualized by stable expression of FASTKD2-eGFP (n= 7 cells). b, Representative time-lapse images of an MRG splitting event in a live COS-7 cell, monitored by SIM. MRGs are visualized by stable expression of FASTKD2-eGFP (n= 6 cells). c, d, Representative time-lapse images of nucleoid “kiss-and-run” (n=1 cell) and splitting events (n=2 cells), respectively, in COS-7 cells, monitored by SIM. Nucleoids are visualized by transient expression of TWINKLE-eGFP and mitochondrial outlines are highlighted by TOMM20-eGFP expression. a–d, Cells were imaged at 1/5 Hz. White arrowheads indicate the dynamic events. Dashed lines indicate the segments used to measure the intensity (grey values) represented in the plots below. Linear Fire LUTs are used to represent pixel intensity values. Scale bars: 2 μm. Statistical source data are provided in Source data Extended data Fig. 4.
Extended Data Fig. 5 CLEM of FASTKD2-tRFP and MRG electron densities.
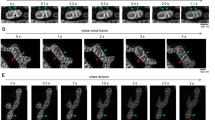
Correlative confocal fluorescence micrograph of FASTKD2-tRFP and transmission electron micrograph (TEM) in COS-7 control (a - c)(7 MRGs were examined from 3 mitochondria of a single cell) and Drp1K38A-CFP positive (d, e) (10 MRGs were examined from 2 mitochondria of a single cell) cells. TEM-highlights correspond to the data presented in Figs. 3a and 4e respectively, and show additional examples. Contiguous 50 nm TEM microtome sections show electron densities corresponding to the MRGs visualized by fluorescence microscopy (yellow arrowheads). Scale bars: Confocal: 10 µm; Confocal zoom: 2 µm; CLEM and TEM sections: 500 nm.
Extended Data Fig. 6 Membrane association and distribution of MRGs within mitochondria.
a, STED microscopy of HeLa cells stably expressing Cox8a-SNAP (grey) and FASTKD2-eGFP (green), treated with 100 µM antimycin A for 1 hour, prior to labelling with SIR-SNAP dye and live cell imaging (The experiment has been performed twice with similar results). A zoomed field of view on the right (dashed box). Scale bar: 2 µm. b-c, FASTKD2-eGFP expressing COS-7 cells untreated (b) or treated with 25 µM antimycin A for 24 hours (c), imaged live using SIM microscopy. The same linear Fire LUT is used for pixel intensity values as in Fig. 2e. Zoomed time-lapse series are shown for two mitochondria (dashed boxes) (The experiment has been performed twice with similar results). Kymographs below (plotted lines are represented on the analysed mitochondria as yellow dashed lines). Scale-bar: 2 µm. d, Example FOV of semi-automated mitochondria segmentation and MRG-association with their parent organelle with the ImageJ plugin, MicrobeJ. (The experiment has been performed three times with similar results). e, and f, Histograms of absolute or relative position of MRGs (n = 231 MRGs examined over 3 independent experiments) within their host mitochondria (green) and simulated, randomly positioned granules (grey). The observed distribution of MRG positions is not significantly different from the simulated random distribution (Kolmogorov-Smirnov test: pabs = 0.057, pnorm = 0.053). Statistical source data are provided in Source data Extended data fig. 6.
Extended Data Fig. 7 Effect of perturbations to mitochondrial dynamics on MRG distribution.
Representative widefield-microscopy images of HeLa cells silenced using siRNAs against Drp1 (second row) or Mfn2 (third row). Cells were fixed after 72 hours of silencing. A negative control siRNA was used in parallel, and is shown in the first row. MRGs and nucleoids were immunolabeled using anti-FASTKD2 (green) and anti-DNA (magenta), respectively. Mitochondria were labelled using MitoTracker Deep Red staining (cyan) (The experiment has been performed twice with similar results). Scale-bar: 10 µm. Disrupted MRG and nucleoid positioning, and clumped appearance as described for mito-bulbs is apparent when either Drp1 and Mfn2 are silenced, but the negative control shows well-dispersed MRGs and nucleoids.
Supplementary information
Supplementary Information
Supplementary discussion and Figs. 1–4.
Supplementary Tables
Supplementary Table 1: Two-colour STORM data. Data for all the individual two-colour mtRNA and FASTKD2 clusters (n = 26) analysed and represented in Fig. 1f and Supplementary Fig. 2. Supplementary Table 2: Summary of fusion events, and association with mitochondrial remodelling.
Supplementary Video 1
FRAP of COS-7 FASTKD2-eGFP. Exemplary movie of FRAP recovery of COS-7 cells stably expressing FASTKD2-eGFP, as shown in Fig. 2a. Time 0 marks the moment of photobleaching. Scale bar, 2 µm.
Supplementary Video 2
FRAP of U2OS FASTKD2-eGFP. Exemplary movie of FRAP recovery of U2OS cells stably expressing FASTKD2-eGFP. Time 0 marks the moment of photobleaching. Scale bar, 2 μm.
Supplementary Video 3
FRAP of COS-7 ERAL1-eGFP. Exemplary movie of FRAP recovery of COS-7 cells stably expressing ERAL1-eGFP, as shown in Fig. 2c. Time 0 marks the moment of photobleaching. Scale bar, 2 μm.
Supplementary Video 4
FRAP of COS-7 DDX28-eGFP. Exemplary movie of FRAP recovery of COS-7 cells stably expressing DDX28-eGFP, as shown in Fig. 2c. Time 0 marks the moment of photobleaching. Scale bar, 2 μm.
Supplementary Video 5
COS-7 FASTKD2-eGFP granule fusion. Exemplary movie of MRG fusion in COS-7 cells stably expressing FASTKD2-eGFP, as shown in Fig. 2e. Scale bar, 2 μm.
Supplementary Video 6
U2OS FASTKD2-eGFP granule fusion. Exemplary movie of MRG fusion in U2OS cells stably expressing FASTKD2-eGFP. Scale bar, 2 μm.
Supplementary Video 7
COS-7 FASTKD2-eGFP granule fission. Exemplary movie of MRG fission in COS-7 cells stably expressing FASTKD2-eGFP. Scale bar, 2 μm.
Supplementary Video 8
COS-7 TWINKLE-eGFP granule fusion. Exemplary movie of nucleoid fusion in COS-7 cells transiently transfected with TWINKLE-eGFP. Scale bar, 2 μm.
Supplementary Video 9
COS-7 TWINKLE-eGFP granule kiss-and-run. Exemplary movie of nucleoid kissing in COS-7 cells transiently transfected with TWINKLE-eGFP. Scale bar, 2 μm.
Supplementary Video 10
COS-7 FASTKD2-eGFP upon 100 µM antimycin A treatment. Exemplary SIM movie of MRGs in swollen mitochondria in COS-7 cells stably expressing FASTKD2-eGFP, after 1 h of 100 µM antimycin A treatment. Scale bar, 2 μm.
Supplementary Video 11
COS-7 FASTKD2-eGFP upon 25 µM antimycin A treatment. Exemplary SIM movie of MRGs in swollen mitochondria in COS-7 cells stably expressing FASTKD2-eGFP, after 1 day (24 h) of 25 µM antimycin A treatment. Scale bar, 2 μm.
Supplementary Video 12
FRAP of COS-7 FASTKD2-tRFP. Exemplary movie of FRAP recovery of COS-7 cells stably expressing FASTKD2-tRFP, as shown in Fig. 4d, c. Time 0 marks the moment of photobleaching. Scale bar, 2 μm.
Supplementary Video 13
FRAP of COS-7 FASTKD2-tRFP with Drp1K38A-YFP. Movie of FRAP recovery of COS-7 cells stably expressing FASTKD2-tRFP, and transfected with DRP1K38A dominant negative mutant as shown in Fig. 4c. Time 0 marks the moment of photobleaching. Scale bar, 2 μm.
Source data
Statistical Source Data Fig. 1
Statistical source data Fig.1b,f.
Statistical Source Data Fig. 2
Statistical source data Fig.2c,d,f.
Statistical Source Data Fig. 3
Statistical source data Fig.3e–g.
Unprocessed Blots Figure 3
Unprocessed western blots.
Statistical Source Data Fig. 4
Statistical source data Fig.4d.
Statistical Source Data Extended Data Fig. 1
Statistical source data Extended Data Fig. 1.
Statistical Source Data Extended Data Fig. 2
Statistical source data Extended Data Fig. 2.
Statistical Source Data Extended Data Fig. 6
Statistical source data Extended Data Fig. 6.
Rights and permissions
About this article
Cite this article
Rey, T., Zaganelli, S., Cuillery, E. et al. Mitochondrial RNA granules are fluid condensates positioned by membrane dynamics. Nat Cell Biol 22, 1180–1186 (2020). https://doi.org/10.1038/s41556-020-00584-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-00584-8
This article is cited by
-
RNA binding protein: coordinated expression between the nuclear and mitochondrial genomes in tumors
Journal of Translational Medicine (2023)
-
Hypoxia-induced mitochondrial stress granules
Cell Death & Disease (2023)
-
Protein nanocondensates: the next frontier
Biophysical Reviews (2023)
-
CryoET reveals organelle phenotypes in huntington disease patient iPSC-derived and mouse primary neurons
Nature Communications (2023)
-
Organization and expression of the mammalian mitochondrial genome
Nature Reviews Genetics (2022)