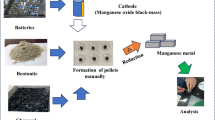
Abstract
The purpose of this work was to explore the incorporation of black manganese oxide powder extracted from alkaline batteries into sintered-clay bricks. The black manganese oxide powder was added into the clay mixture in two different states: pristine and pre-vitrified. Three levels of addition were evaluated according to the final wt% of manganese in the bricks: 0.0, 0.1, and 2.5. The resulting test-bricks were then assessed to determine the following physical and mechanical properties: color change, water absorption, water saturation coefficient, compressive strength, and leaching/efflorescence tendency. The results were statistically tested for significance and compared to a standard industrial formulation for non-structural sintered-clay bricks used locally in Girón, Santander (Colombia). In general, the properties of the test-bricks deviated slightly from the standard formulation only when the final content of manganese in the test-bricks reached 2.5 wt%. In particular, the test-bricks with 2.5 wt% of manganese added in the pre-vitrified state, showed a darker brown color, 1.7 % less water absorption, and a 68 % increase on compressive strength, when compared to the industrial reference red-clay bricks. It is worth to point out that none of the test-bricks studied showed efflorescence tendency. In conclusion, the work proves that the Mn-oxides contained in spent alkaline batteries can be successfully incorporated into nonstructural red clay bricks. These findings would help to ease the adoption of novel circular economy strategies in developing countries with no access to metallurgical facilities for battery recycling.
Graphic Abstract















Similar content being viewed by others
References
Viczek, S.A., Aldrian, A., Pomberger, R., Sarc, R.: Origins and carriers of Sb, As, Cd, Cl, Cr Co, Pb, Hg, and Ni in mixed solid waste—a literature-based evaluation. Waste Manag. 103, 87–112 (2020). https://doi.org/10.1016/j.wasman.2019.12.009
Tetsopgang, S., Kuepouo, G.: Quantification and characterization of discarded batteries in Yaoundé, from the perspective of health, safety and environmental protection. Resour. Conserv. Recycl. 52, 1077–1081 (2008). https://doi.org/10.1016/j.resconrec.2008.04.006
Daryabeigi Zand, A., Abduli, M.A.: Current situation of used household batteries in Iran and appropriate management policies. Waste Manag. 28, 2085–2090 (2008). https://doi.org/10.1016/j.wasman.2007.09.013
Zambrano Colmenares, A., Romero Briceño, C.H., Moccia Paradisi, A., Orta Rodríguez, R.E., López Sanz, J.L., Delvasto Angarita, P.: Hydrometallurgical valuing of cathodic and anodic materials of used rechargeable batteries from the Ni-MH Type. Prod. + Limpia. 10, 51–63 (2015)
Ferronato, N., Torretta, V.: Waste mismanagement in developing countries: A review of global issues. Int. J. Environ. Res. Public Health. 16, 1–28 (2019). https://doi.org/10.3390/ijerph16061060
Slack, R.J., Gronow, J.R., Voulvoulis, N.: Household hazardous waste in municipal landfills: Contaminants in leachate. Sci. Total Environ. 337, 119–137 (2005). https://doi.org/10.1016/j.scitotenv.2004.07.002
Karnchanawong, S., Limpiteeprakan, P.: Evaluation of heavy metal leaching from spent household batteries disposed in municipal solid waste. Waste Manag. 29, 550–558 (2009). https://doi.org/10.1016/j.wasman.2008.03.018
Godoy, K.A.M., Delvasto, P.L.: Quantification of the metals Ni, Cd, Zn, Mn, Fe and Co in leachates during degradation of three types of used commercial batteries (alkaline, Ni–Cd AND Ni–MH). Rev. Invest. (Caracas) 39, 131–156 (2015)
Godoy, K.A.M., Delvasto, P.L.: Soil pollution by heavy metals due to the presence of spent batteries. Rev. Invest. (Caracas) 88, 78–104 (2016)
Ogundele, L.T., Ayeku, P.O., Adebayo, A.S., Olufemi, A.P., Adejoro, I.A.: Pollution indices and potential ecological risks of heavy metals in the soil: A case study of municipal wastes site in Ondo State, Southwestern. Nigeria. Polytechnica. (2020). https://doi.org/10.1007/s41050-020-00022-6
Espinosa, D.C.R., Bernardes, A.M., Tenório, J.A.S.: An overview on the current processes for the recycling of batteries. J. Power Sources. 135, 311–319 (2004). https://doi.org/10.1016/j.jpowsour.2004.03.083
Hagelüken, C., Lee-Shin, J.U., Carpentier, A., Heron, C.: The EU circular economy and its relevance to metal recycling. Recycling. 1, 242–253 (2016). https://doi.org/10.3390/recycling1020242
Nogueira, C.A., Margarido, F.: Battery recycling by hydrometallurgy: Evaluation of simultaneous treatment of several cell systems. In: Salazar-Villalpando, M.D., Neelameggham, N.R., Guillen, D.P., Pati, S., Krumdick, G.K. (eds.) Energy Technol 2012: Carbon dioxide management and other technologies. Wiley, Hoboken USA (2012)
Assefi, M., Maroufi, S., Yamauchi, Y., Sahajwalla, V.: Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 24, 26–31 (2020). https://doi.org/10.1016/j.cogsc.2020.01.005
Bernardes, A.M., Espinosa, D.C.R., Tenório, J.A.S.: Recycling of batteries: A review of current processes and technologies. J. Power Sources. 130, 291–298 (2004). https://doi.org/10.1016/j.jpowsour.2003.12.026
Maryam Sadeghi, S., Jesus, J., Soares, H.M.V.M.: A critical updated review of the hydrometallurgical routes for recycling zinc and manganese from spent zinc-based batteries. Waste Manag. 113, 342–350 (2020). https://doi.org/10.1016/j.wasman.2020.05.049
Sobianowska-Turek, A., Szczepaniak, W., Maciejewski, P., Gawlik-Kobylińska, M.: Recovery of zinc and manganese, and other metals (Fe, Cu, Ni Co, Cd, Cr, Na, K) from Zn–MnO2 and Zn–C waste batteries: Hydroxyl and carbonate co-precipitation from solution after reducing acidic leaching with use of oxalic acid. J. Power Sources. 325, 220–228 (2016). https://doi.org/10.1016/j.jpowsour.2016.06.042
Gollakota, A.R.K., Gautam, S., Shu, C.M.: Inconsistencies of e-waste management in developing nations—facts and plausible solutions. J. Environ. Manage. 261, 110234 (2020). https://doi.org/10.1016/j.jenvman.2020.110234
Pistoia, G., Wiaux, J.-P., Wolsky, S.P.: Used battery collection and recycling. Elsevier, Amsterdam (2001)
Espinosa, D.C.R., Bernardes, A.M., Tenório, J.A.S.: Brazilian policy on battery disposal and its practical effects on battery recycling. J. Power Sources. 137, 134–139 (2004). https://doi.org/10.1016/j.jpowsour.2004.02.023
Ministry of the Environment Housing and Territorial Development (Republic of Colombia): Resolution 1297 (2010), https://www.habitatbogota.gov.co/resolucion-1297 (In Spanish)
National Institute for Industrial Technology (INTI): Electrical battery management in Argentina (2016), https://www.inti.gob.ar/ambientesg/pdf/pilasybaterias2016.pdf (In Spanish)
Ministry of the Interior and Public Security (Republic of Chile): Law 20.923 (2016), https://portal.mma.gob.cl/wp-content/uploads/2015/06/do-20160601-web.pdf (In Spanish)
Savino, A., Solorzano, G., Quispe, C., Correal, M.C.: Waste management outlook for Latin America and the Caribbean. United Nations Environment Programme, Argentina, 9 October 2018
Local Environmental Authority of the Cauca Valley Region (Republic of Colombia): Resolution 0100 No.0150–0895 (2016), https://ecotecsas.com/wp-content/uploads/2016/08/LICENCIA-ECOTEC-SAS.pdf (In Spanish)
Tanong, K., Tran, L.H., Mercier, G., Blais, J.F.: Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 148, 233–244 (2017). https://doi.org/10.1016/j.jclepro.2017.01.158
Colmenares, A.Z., Salaverría, J.D., Delvasto, P.: Characterization of the chemical compounds obtained after using acetic acid as leaching agent in the hydrometallurgical treatment of spent Ni–MH batteries. Prod. + Limpia. 13, 19–29 (2018). https://doi.org/10.22507/pml.v13n1a2
Molina-Silva, J., Vera-Serna, P., Delvasto, P.: Characterization of the metallic phase and the slag phase obtained during the inertization of the cathodes of spent alkaline batteries using liquid aluminum. J. Phys. Conf. Ser. (2019). https://doi.org/10.1088/1742-6596/1386/1/012056
Bertuol, D.A., Amado, F.D.R., Veit, H., Ferreira, J.Z., Bernardes, A.M.: Recovery of nickel and cobalt from spent NiMH batteries by electrowinning. Chem. Eng. Technol. 35, 2084–2092 (2012). https://doi.org/10.1002/ceat.201200283
Diáz-López, J.C., Angarita, J., Vargas-Angarita, C.Y., Blanco, S., Delvasto, P.: Electrolytic recovery of nickel and cobalt as multi-elemental coatings: An option for the recycling of spent Ni–MH batteries. J. Phys. Conf. Ser. (2018). https://doi.org/10.1088/1742-6596/1119/1/012003
Quiroz, D., Pinto, J., Blanco, S., Delvasto, P.: Synthesis and characterization of Zn/Ni–Co bilayer coatings using the metals recovered from spent household batteries as raw materials. J. Phys. Conf. Ser. (2018). https://doi.org/10.1088/1742-6596/1119/1/012007
Pinto, J., Quiroz, D., Delvasto, P., Blanco, S.: Characterization of a zinc-nickel alloy coating obtained from an electrolytic bath produced with spent batteries as raw materials. J. Phys. Conf. Ser. (2018). https://doi.org/10.1088/1742-6596/1119/1/012005
Forero, B.J., Díaz-Salaverría, J.V., Delvasto, P., Gouveia, C.X.: Leaching characteristics of co-bearing glasses obtained from spent Li-ion batteries. In: Vilarinho, C. (ed.) WASTES–solutions, treatments and opportunities II–selected papers from the 4th edition of the International Conference Wastes: Solutions, Treatments and Opportunities, pp. 17–22. CRC Press, Boca Raton (2018)
Assías, S.G., Pabón, F., Cala, N., Delvasto, P.: Incorporation of fluorescent lamp waste into red-clay bricks: Defect formation, physical and mechanical properties. Waste and Biomass Valorization. (2020). https://doi.org/10.1007/s12649-020-01075-5
Monteiro, S.N., Vieira, C.M.F.: On the production of fired clay bricks from waste materials: A critical update. Constr. Build. Mater. 68, 599–610 (2014). https://doi.org/10.1016/j.conbuildmat.2014.07.006
Singh, H., Brar, G.S., Mudahar, G.S.: Evaluation of characteristics of fly ash-reinforced clay bricks as building material. J. Build. Phys. 40, 1–14 (2016). https://doi.org/10.1177/1744259116659662
Babisk, M.P., Amaral, L.F., Silva Ribeiro, L., Vieira, C.M., Prado, U.S., Gadioli, M.C., Oliveira, M.S., da Luz, F.S., Monteiro, S.N., da Costa Garcia Filho, F.: Evaluation and application of sintered red mud and its incorporated clay ceramics as materials for building construction. J. Mater. Res. Technol. 9, 2186–2195 (2019). https://doi.org/10.1016/j.jmrt.2019.12.049
Vieira, C.M.F., Sanchez, R., Monteiro, S.N., Lalla, N., Quaranta, N.: Recycling of electric arc furnace dust into red ceramic. J. Mater. Res. Technol. 2, 88–92 (2013). https://doi.org/10.1016/j.jmrt.2012.09.001
Zhang, M., Chen, C., Mao, L., Wu, Q.: Use of electroplating sludge in production of fired clay bricks: Characterization and environmental risk evaluation. Constr. Build. Mater. 159, 27–36 (2018). https://doi.org/10.1016/j.conbuildmat.2017.10.130
Shih, P.H., Wu, Z.Z., Chiang, H.L.: Characteristics of bricks made from waste steel slag. Waste Manag. 24, 1043–1047 (2004). https://doi.org/10.1016/j.wasman.2004.08.006
Andreola, F., Barbieri, L., Lancellotti, I., Leonelli, C., Manfredini, T.: Recycling of industrial wastes in ceramic manufacturing: State of art and glass case studies. Ceram. Int. 42, 13333–13338 (2016). https://doi.org/10.1016/j.ceramint.2016.05.205
Morais, A.S.C., Vieira, C.M.F., Rodriguez, R.J.S., Monteiro, S.N., Candido, V.S., Ferreira, C.L.: Fluorescent lamp glass waste incorporation into clay ceramic: A perfect solution. Jom. 68, 2425–2434 (2016). https://doi.org/10.1007/s11837-016-1985-z
Abramov, O., Medvedev, A., Tomashevskaya, N.: TRIZ Roadmap for Identifying Adjacent Markets Oleg. In: Souchkov, S. and Mayer, O. (eds.) The 13th international conference TRIZ fest. pp. 382–390. Heilbronn, Germany Proceedings (2019)
Rodrigues Fiuza, T.E., Göttert, D., Pereira, L.J., Masetto Antunes, S.R., de Chaves Andrade, A.V., Antunes, A.C., de Ferreira Souza, É.C.: Production of brown inorganic pigments with spinel structure using spent zinc-carbon batteries. Process. Appl. Ceram. 12, 319–325 (2018). https://doi.org/10.2298/PAC1804319R
Im, H. hag: Method for making ceramic-coloring clay bricks using waste batteries powder. Patent US 2010/0090377 A1, (2010)
Delvasto, P.L., Niño-Avendaño, C., Moreno, I.: Urban mining: spent batteries as a metalliferous resource. In: CIM 2015–VIII Congreso Internacional de Materiales. Paipa, Columbia, 28–30 October 2015
American Society for Testing and Materials: ASTM C702–18 standard practice for reducing samples of aggregate to testing size. ASTM International, West Conshocken (2018)
Ferella, F., De Michelis, I., Vegliò, F.: Process for the recycling of alkaline and zinc-carbon spent batteries. J. Power Sources. 183, 805–811 (2008). https://doi.org/10.1016/j.jpowsour.2008.05.043
Belardi, G., Ballirano, P., Ferrini, M., Lavecchia, R., Medici, F., Piga, L., Scoppettuolo, A.: Characterization of spent zinc-carbon and alkaline batteries by SEM-EDS. TGA/DTA and XRPD analysis. Thermochim. Acta. 526, 169–177 (2011). https://doi.org/10.1016/j.tca.2011.09.012
Loryuenyong, V., Panyachai, T., Kaewsimork, K., Siritai, C.: Effects of recycled glass substitution on the physical and mechanical properties of clay bricks. Waste Manag. 29, 2717–2721 (2009). https://doi.org/10.1016/j.wasman.2009.05.015
Goñi, S.M., Salvadori, V.O.: Color measurement: comparison of colorimeter vs. computer vision system. J. Food Meas. Charact. 11, 538–547 (2017). https://doi.org/10.1007/s11694-016-9421-1
Soldat, D.J., Barak, P., Lepore, B.J.: Microscale colorimetric analysis using a desktop scanner and automated digital image analysis. J. Chem. Educ. 86, 617–620 (2009). https://doi.org/10.1021/ed086p617
Ferreira, T., Rasband, W.: ImageJ User Guide, https://imagej.nih.gov/ij/index.html
ASTM: Standard practice for calculation of color tolerances and color differences from instrumentally measured color coordinates. ASTM International, 93, 1–13. West Conshohocken, PA (2003).
Mclaren, K., Taylor, P.F.: The derivation of hue-difference terms from CIELAB coordinates. Color Res. Appl. 6, 75–77 (1981). https://doi.org/10.1002/col.5080060207
ICONTEC: NTC (Colombian Technical Standard) 4017 Method of Sampling and Test of Masonry Units and other Clay Products, (2005)
Madejová, J.: FTIR techniques in clay mineral studies. Vib. Spectrosc. 31, 1–10 (2003). https://doi.org/10.1016/S0924-2031(02)00065-6
Nayak, P.S., Singh, B.K.: Instrumental characterization of clay by FTIR, XRF, BET and TPD-NH3. Bull. Mater. Sci. 30, 235–238 (2007)
Tiffo, E., Elimbi, A., Manga, J.D., Tchamba, A.B.: Red ceramics produced from mixtures of kaolinite clay and waste glass. Brazilian J. Sci. Technol. (2015). https://doi.org/10.1186/s40552-015-0009-9
Madejová, J., Gates, W.P., Petit, S.: IR spectra of clay minerals. In: Gates, W. (ed.) Developments in clay science, pp. 107–149. Elsevier, Amsterdam (2017)
Beran, A., Libowitzky, E.: Spectroscopic methods in Mineralogy. The Mineralogical Society of Great Britain and Ireland (2004). https://doi.org/10.1180/EMU-notes.6
Hu, P., Yang, H.: Insight into the physicochemical aspects of kaolins with different morphologies. Appl. Clay Sci. 74, 58–65 (2013). https://doi.org/10.1016/j.clay.2012.10.003
Socrates, G.: Infrared and Raman characteristic group frequencies: Tables and charts. Wiley, Hoboken, USA (2001)
Friedel, R.A., Queiser, J.A.: Infrared and Raman Spectra of intractable carbonaceous substances—reassignments I N coal spectra. Am. Chem. Soc., Div. Fuel Chem. 1, 123–136 (1971)
Narita, E., Okabe, T.: The formation and some properties of hydrous manganese (IV) oxide. Bull. Chem. Soc. Jpn. 53, 525–532 (1979)
Kupracz, P., Karczewski, J., Przëniak-Welenc, M., Szreder, N.A., Winiarski, M.J., Klimczuk, T., Barczy’nski, R.J.: Microstructure and electrical properties of manganese borosilicate glasses. J. Non. Cryst. Solids. 423–424, 68–75 (2015). https://doi.org/10.1016/j.jnoncrysol.2015.05.014
Zaitizila, I., Halimah, M.K., Muhammad, F.D., Nurisya, M.S.: Influence of manganese doping on elastic and structural properties of silica borotellurite glass. J. Non. Cryst. Solids. 492, 50–55 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.04.019
Wang, S.T., Chen, M.L., Feng, Y.Q.: A meso-macroporous borosilicate monolith prepared by a sol-gel method. Microporous Mesoporous Mater. 151, 250–254 (2012). https://doi.org/10.1016/j.micromeso.2011.10.029
Kaky, K.M., Lakshminarayana, G., Baki, S.O., Taufiq-Yap, Y.H., Kityk, I.V., Mahdi, M.A.: Structural, thermal, and optical analysis of zinc boro-aluminosilicate glasses containing different alkali and alkaline modifier ions. J. Non. Cryst. Solids. 456, 55–63 (2017). https://doi.org/10.1016/j.jnoncrysol.2016.10.044
Gautam, C., Yadav, A.K., Singh, A.K.: A review on Infrared Spectroscopy of borate glasses with effects of different additives. ISRN Ceram. 2012, 1–17 (2012). https://doi.org/10.5402/2012/428497
Coletti, C., Maritan, L., Cultrone, G., Dalconi, M.C., Hein, A., Molina, E., Mazzoli, C.: Recycling trachyte waste from the quarry to the brick industry: Effects on physical and mechanical properties, and durability of new bricks. Constr. Build. Mater. 166, 792–807 (2018). https://doi.org/10.1016/j.conbuildmat.2018.01.158
Hwang, J.-Y., Huang, X., Garkida, A., Hein, A.: Waste colored glasses as sintering aid in ceramic tiles production. J. Miner. Mater. Charact. Eng. 05, 119–129 (2006). https://doi.org/10.4236/jmmce.2006.52008
Federico, L., Chidiac, S.E., Drysdale, R.G.: The use of waste material in the manufacturing of clay brick. In: 10th Canadian Masonry Symposium. Banff, Alberta (2005)
Molera, J., Coll, J., Labrador, A., Pradell, T.: Manganese brown decorations in 10th to 18th century Spanish tin glazed ceramics. Appl. Clay Sci. 82, 86–90 (2013). https://doi.org/10.1016/j.clay.2013.05.018
Chidiac, S.E., Federico, L.M.: Effects of waste glass additions on the properties and durability of fired clay brick. Can. J. Civ. Eng. 34, 1458–1466 (2007). https://doi.org/10.1139/L07-120
Netinger, I., Vračević, M., Ranogajec, J., Vučetić, S.: Evaluation of brick resistance to freeze/thaw cycles according to indirect procedures procedures. Gradjevinar. 66, 197–209 (2014). https://doi.org/10.14256/JCE.956.2013
European Commission: International Chemical Safety Cards (ICSCs): Potassium hydroxide, https://www.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0357&p_version=2, (2017)
European Commission: International Chemical Safety Cards (ICSCs): Potassium oxide, https://www.inchem.org/documents/icsc/icsc/eics0769.htm, (2017)
Donald, I.W.: Waste immobilization in glass and ceramic based hosts: Radioactive, toxic, and hazardous wastes. Wiley, Wiltshire (2010)
Phonphuak, N., Kanyakam, S., Chindaprasirt, P.: Utilization of waste glass to enhance physical-mechanical properties of fired clay brick. J. Clean. Prod. 112, 3057–3062 (2016). https://doi.org/10.1016/j.jclepro.2015.10.084
Mehta, M., Scarborough, W., Armpriest, D.: Building construction: Principles, materials, and systems. Pearson, New Jersey (2013)
Meille, S., Lombardi, M., Chevalier, J., Montanaro, L.: Mechanical properties of porous ceramics in compression: On the transition between elastic, brittle, and cellular behavior. J. Eur. Ceram. Soc. 32, 3959–3967 (2012). https://doi.org/10.1016/j.jeurceramsoc.2012.05.006
Nelson, L.O., Kong, P., Anderson, G., Choi, K., Kim, C.W., Shin, S.W.: Vitrification of Korean Low-Level Waste. In: Sundaram, S.K., Spearing, D.R., Vienna, J.D. (eds.) Proceedings of the Science and Technology in Addressing Environmetal Issues in the Ceramic Industry and Ceramic Science and Technology for the Nuclear Industry symposia held at the 104th Annual Meeting of The American Ceramic Society, pp. 177–184. The American Ceramic Society, St. Louis (2003)
Paul, A.: Chemistry of glasses. Springer, Dordrecht (1982)
U.S. Enviromental Protection Agency: Handbook of vitrification technologies for treatment of hazardous and radioactive waste (EPA/625/R-92/002). Bibliogov, Cincinnati (1992)
Brocken, H., Nijland, T.G.: White efflorescence on brick masonry and concrete masonry blocks, with special emphasis on sulfate efflorescence on concrete blocks. Constr. Build. Mater. 18, 315–323 (2004). https://doi.org/10.1016/j.conbuildmat.2004.02.004
Pel, L., Huinink, H., Kopinga, K., Van Hees, R.P.J., Adan, O.C.G.: Efflorescence pathway diagram: Understanding salt weathering. Constr. Build. Mater. 18, 309–313 (2004). https://doi.org/10.1016/j.conbuildmat.2004.02.003
Buxbaum, G., Pfaff, G.: Industrial inorganic pigments. Wiley, Weinheim (2005)
Superintendence of Domiciliary Public Services (Republic of Colombia): National report on the reclaiming of solid waste (2017), https://www.andi.com.co/Uploads/22. Informa de Aprovechamiento 187302.pdf (In Spanish)
Acknowledgements
The authors would like to thank Miss. Cassandra Dewan and Mr. Wai Liam Ng for the thorough revisions and useful comments on the draft version of the manuscript. Mr. Ambrosio Carrillo is also acknowledged for his technical support during the experimental part of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Assías, S.G., Clavijo, C., Usma, S. et al. On the Incorporation of Pristine and Pre-vitrified Alkaline Battery Waste into Non-structural Clay Bricks. Waste Biomass Valor 12, 3589–3604 (2021). https://doi.org/10.1007/s12649-020-01259-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01259-z