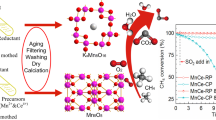
Abstract
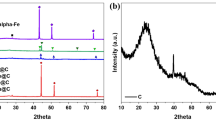
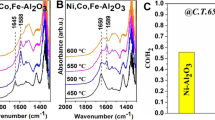
Transition metal (Ni, Cu and Fe) substituted Co3O4–ZrO2 catalysts (NiCZ, CuCZ and FeCZ, respectively) were synthesized by PEG assisted sonochemical synthesis and tested for lean methane combustion. These catalysts offered complete combustion of methane by generating CO2 and steam as products. The strong metal-support interaction (SMSI) allowed the high catalytic activity at temperatures below 600 °C. All catalysts have shown superior stability for 20 h of time on stream conditions. In practical conditions, SO2 presence is obvious in methane feed, even trace levels of it inhibits the catalytic activity to a greater extent. In this study, the performance of catalysts and reaction mechanism was evaluated in presence of SO2 in reactant feed using packed bed catalytic activity studies and in situ FTIR. Among all catalysts, FeCZ has shown superior catalytic activity even under SO2 in the feed compared to NiCZ and CuCZ. The apparent activation energy was found to be larger in case of SO2 presence for all the catalysts. The structural and reducibility properties have been characterized with the XRD, XPS, TEM and H2–TPR studies.









Similar content being viewed by others
References
Wei L, Geng P (2016) A review on natural gas/diesel dual fuel combustion, emissions and performance. Fuel Process Technol 142:264–278. https://doi.org/10.1016/j.fuproc.2015.09.018
Gélin P, Urfels L, Primet M, Tena E (2003) Complete oxidation of methane at low temperature over Pt and Pd catalysts for the abatement of lean-burn natural gas fuelled vehicles emissions: influence of water and sulphur containing compounds. Catal Today 83(1):45–57. https://doi.org/10.1016/S0920-5861(03)00215-3
Kakaee A-H, Paykani A, Ghajar M (2014) The influence of fuel composition on the combustion and emission characteristics of natural gas fueled engines. Renew Sustain Energy Rev 38:64–78. https://doi.org/10.1016/j.rser.2014.05.080
Fernández J, Marín P, Díez FV, Ordóñez S (2015) Coal mine ventilation air methane combustion in a catalytic reverse flow reactor: Influence of emission humidity. Fuel Process Technol 133:202–209. https://doi.org/10.1016/j.fuproc.2015.02.005
Gosiewski K, Pawlaczyk A (2014) Catalytic or thermal reversed flow combustion of coal mine ventilation air methane: what is better choice and when? Chem Eng J 238:78–85. https://doi.org/10.1016/j.cej.2013.07.039
Eyssler A, Mandaliev P, Winkler A, Hug P, Safonova O, Figi R, Weidenkaff A, Ferri D (2010) The effect of the state of Pd on methane combustion in Pd-Doped LaFeO3. J Phys Chem C 114(10):4584–4594. https://doi.org/10.1021/jp911052s
Specchia S, Conti F, Specchia V (2010) Kinetic studies on Pd/CexZr1−xO2 catalyst for methane combustion. Ind Eng Chem Res 49(21):11101–11111. https://doi.org/10.1021/ie100532x
Guo X, Zhi G, Yan X, Jin G, Guo X, Brault P (2011) Methane combustion over Pd/ZrO2/SiC, Pd/CeO2/SiC, and Pd/Zr0.5Ce0.5O2/SiC catalysts. Catal. Commun. 12(10):870–874. https://doi.org/10.1016/j.catcom.2011.02.007
Di Carlo G, Melaet G, Kruse N, Liotta LF, Pantaleo G, Venezia AM (2010) Combined sulfating and non-sulfating support to prevent water and sulfur poisoning of Pd catalysts for methane combustion. Chem Commun 46(34):6317–6319. https://doi.org/10.1039/C0CC00723D
Yang N, Liu J, Sun Y, Zhu Y (2017) Au@PdOx with a PdOx-rich shell and Au-rich core embedded in Co3O4 nanorods for catalytic combustion of methane. Nanoscale 9(6):2123–2128. https://doi.org/10.1039/C6NR08700K
Colussi S, Arosio F, Montanari T, Busca G, Groppi G, Trovarelli A (2010) Study of sulfur poisoning on Pd/Al2O3 and Pd/CeO2/Al2O3 methane combustion catalysts. Catal Today 155(1):59–65. https://doi.org/10.1016/j.cattod.2009.02.019
Xu J, Ouyang L, Mao W, Yang X-J, Xu X-C, Su J-J, Zhuang T-Z, Li H, Han Y-F (2012) Operando and kinetic study of low-temperature, lean-burn methane combustion over a Pd/γ-Al2O3 catalyst. ACS Catal 2(2):261–269. https://doi.org/10.1021/cs200694k
Li G, Hu W, Huang F, Chen J, Gong M, Yuan S, Chen Y, Zhong L (2017) Pd catalyst supported on ZrO2-Al2O3 by double-solvent method for methane oxidation under lean conditions. Can J Chem Eng 95(6):1117–1123. https://doi.org/10.1002/cjce.22750
Xu J, Li P, Song X, He C, Yu J, Han Y-F (2010) Operando Raman spectroscopy for determining the active phase in one- dimensional Mn1 − xCexO2±y nanorod catalysts during methane combustion. J Phys Chem Lett 1(10):1648–1654. https://doi.org/10.1021/jz1004522
Li H, Lu G, Qiao D, Wang Y, Guo Y, Guo Y (2011) Catalytic methane combustion over Co3O4/CeO2 composite oxides prepared by modified citrate sol-gel method. Catal Lett 141(3):452–458. https://doi.org/10.1007/s10562-010-0513-y
Li X, Liu Y, Deng J, Xie S, Zhao X, Zhang Y, Zhang K, Arandiyan H, Guo G, Dai H (2017) Enhanced catalytic performance for methane combustion of 3DOM CoFe2O4 by co-loading MnOx and Pd–Pt alloy nanoparticles. Appl Surf Sci 403:590–600. https://doi.org/10.1016/j.apsusc.2017.01.237
Wang Z, Deng J, Liu Y, Yang H, Xie S, Wu Z, Dai H (2017) Three-dimensionally ordered macroporous CoCr2O4-supported Au–Pd alloy nanoparticles: highly active catalysts for methane combustion. Catal Today 281:467–476. https://doi.org/10.1016/j.cattod.2016.05.035
Sutthiumporn K, Maneerung T, Kathiraser Y, Kawi S (2012) CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi Co, Cr, Cu, Fe): Roles of lattice oxygen on C-H activation and carbon suppression. Int. J. Hydrogen Energy 37(15):11195–11207. https://doi.org/10.1016/j.ijhydene.2012.04.059
Singh SA, Madras G (2016) Sonochemical synthesis of Pt, Ru doped TiO2 for methane reforming. Appl Catal A: Gen 518:102–114. https://doi.org/10.1016/j.apcata.2015.10.047
Tang M, Liu K, Roddick DM, Fan M (2018) Enhanced lattice oxygen reactivity over Fe2O3/Al2O3 redox catalyst for chemical-looping dry (CO2) reforming of CH4: synergistic La-Ce effect. J Catal 368:38–52. https://doi.org/10.1016/j.jcat.2018.09.022
Hu S, He L, Wang Y, Su S, Jiang L, Chen Q, Liu Q, Chi H, Xiang J, Sun L (2016) Effects of oxygen species from Fe addition on promoting steam reforming of toluene over Fe–Ni/Al2O3 catalysts. Int J Hydrogen Energy 41(40):17967–17975. https://doi.org/10.1016/j.ijhydene.2016.07.271
Weiss W, Schlögl R (2000) An integrated surface science approach towards metal oxide catalysis. Top Catal 13(1):75–90. https://doi.org/10.1023/A:1009041107437
Papavasiliou J, Paxinou A, Słowik G, Neophytides S, Avgouropoulos G (2018) Steam reforming of methanol over nanostructured Pt/TiO2 and Pt/CeO2 catalysts for fuel cell applications. Catalysts. https://doi.org/10.3390/catal8110544
Chen A, Guo H, Song Y, Chen P, Lou H (2017) Recyclable CeO2–ZrO2 and CeO2–TiO2 mixed oxides based Pt catalyst for aqueous-phase reforming of the low-boiling fraction of bio-oil. Int J Hydrogen Energy 42(15):9577–9588. https://doi.org/10.1016/j.ijhydene.2017.03.092
Singh SA, Vishwanath K, Madras G (2017) Role of hydrogen and oxygen activation over Pt and Pd-doped composites for catalytic hydrogen combustion. ACS Appl Mater Interfaces 9(23):19380–19388. https://doi.org/10.1021/acsami.6b08019
Singh SA, Madras G (2015) Detailed mechanism and kinetic study of CO oxidation on cobalt oxide surfaces. Appl Catal A: Gen 504:463–475. https://doi.org/10.1016/j.apcata.2014.10.024
Hegde MS, Madras G, Patil KC (2009) Noble metal ionic catalysts. Acc Chem Res 42(6):704–712. https://doi.org/10.1021/ar800209s
Baidya T, Gupta A, Deshpandey PA, Madras G, Hegde MS (2009) High oxygen storage capacity and high rates of CO oxidation and NO reduction catalytic properties of Ce1−xSnxO2 and Ce0.78Sn0.2Pd0.02O2-δ. J. Phys. Chem. C 113(10):4059–4068. https://doi.org/10.1021/jp8060569
Roy S, Viswanath B, Hegde MS, Madras G (2008) Low-Temperature Selective Catalytic Reduction of NO with NH3 over Ti0.9M0.1O2-δ (M = Cr, Mn, Fe, Co, Cu). J. Phys. Chem. C 112 (15):6002–6012. doi:https://doi.org/10.1021/jp7117086
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32(5):751–767. https://doi.org/10.1107/S0567739476001551
Varun Y, Sreedhar I, Singh SA (2020) Highly stable M/NiO–MgO (M = Co, Cu and Fe) catalysts towards CO2 methanation. Int. J. Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2020.07.212
van Vegten N, Baidya T, Krumeich F, Kleist W, Baiker A (2010) Flame-made MgAl2−xMxO4 (M=Mn, Fe, Co) mixed oxides: Structural properties and catalytic behavior in methane combustion. Appl Catal B: Environ 97(3):398–406. https://doi.org/10.1016/j.apcatb.2010.04.026
Ercolino G, Stelmachowski P, Grzybek G, Kotarba A, Specchia S (2017) Optimization of Pd catalysts supported on Co3O4 for low-temperature lean combustion of residual methane. Appl Catal B: Environ 206:712–725. https://doi.org/10.1016/j.apcatb.2017.01.055
Kwak BS, Park N-K, Ryu SO, Baek J-I, Ryu H-J, Kang M (2017) Improved reversible redox cycles on MTiOx (M=Fe Co, Ni, and Cu) particles afforded by rapid and stable oxygen carrier capacity for use in chemical looping combustion of methane. Chem Eng J 309:617–627. https://doi.org/10.1016/j.cej.2016.10.040
Chen Z, Wang S, Ding Y, Zhang L, Lv L, Wang M, Wang S (2017) Pd catalysts supported on Co3O4 with the specified morphologies in CO and CH4 oxidation. Appl Catal A: Gen 532:95–104. https://doi.org/10.1016/j.apcata.2016.12.021
Hu W, Li G, Chen J, Huang F, Gong M, Zhong L, Chen Y (2017) Enhancement of activity and hydrothermal stability of Pd/ZrO2-Al2O3 doped by Mg for methane combustion under lean conditions. Fuel 194:368–374. https://doi.org/10.1016/j.fuel.2016.11.028
Pu Z, Liu Y, Zhou H, Huang W, Zheng Y, Li X (2017) Catalytic combustion of lean methane at low temperature over ZrO2-modified Co3O4 catalysts. Appl Surf Sci 422:85–93. https://doi.org/10.1016/j.apsusc.2017.05.231
Gao Q-X, Wang X-F, Di J-L, Wu X-C, Tao Y-R (2011) Enhanced catalytic activity of α-Fe2O3 nanorods enclosed with 110 and 001 planes for methane combustion and CO oxidation. Catal Sci Technol 1(4):574–577. https://doi.org/10.1039/C1CY00080B
Khromova SA, Smirnov AA, Bulavchenko OA, Saraev AA, Kaichev VV, Reshetnikov SI, Yakovlev VA (2014) Anisole hydrodeoxygenation over Ni–Cu bimetallic catalysts: the effect of Ni/Cu ratio on selectivity. Appl Catal A: Gen 470:261–270. https://doi.org/10.1016/j.apcata.2013.10.046
Gupta A, Waghmare UV, Hegde MS (2010) Correlation of oxygen storage capacity and structural distortion in transition-metal-, noble-metal-, and rare-earth-ion-substituted CeO2 from first principles calculation. Chem Mater 22(18):5184–5198. https://doi.org/10.1021/cm101145d
Alalwan HA, Cwiertny DM, Grassian VH (2017) Co3O4 nanoparticles as oxygen carriers for chemical looping combustion: a materials characterization approach to understanding oxygen carrier performance. Chem Eng J 319:279–287. https://doi.org/10.1016/j.cej.2017.02.134
Zhang F, Yao S, Liu Z, Gutiérrez RA, Vovchok D, Cen J, Xu W, Ramírez PJ, Kim T, Senanayake SD, Rodriguez JA (2018) Reaction of methane with MOx/CeO2 (M = Fe, Ni, and Cu) catalysts: in situ studies with time-resolved x-ray diffraction. J. Phys. Chem. C 122 (50):28739–28747. https://doi.org/10.1021/acs.jpcc.8b09319
Chen J, Shi W, Li J (2011) Catalytic combustion of methane over cerium-doped cobalt chromite catalysts. Catal Today 175(1):216–222. https://doi.org/10.1016/j.cattod.2011.03.061
Farrauto RJ (2012) Low-temperature oxidation of methane. Science 337(6095):659–660. https://doi.org/10.1126/science.1226310
Ercolino G, Stelmachowski P, Kotarba A, Specchia S (2017) Reactivity of mixed iron-cobalt spinels in the lean methane combustion. Catal Top. https://doi.org/10.1007/s11244-017-0826-9
Shao C, Li W, Lin Q, Huang Q, Pi D (2017) Low temperature complete combustion of lean methane over cobalt-nickel mixed-oxide catalysts. Energy Technol 5(4):604–610. https://doi.org/10.1002/ente.201600402
Peng Y, Za J, Chen J (2017) Mechanism and kinetics of methane combustion, Part I: thermal rate constants for hydrogen-abstraction reaction of CH4 + O(3P). J Phys Chem A 121(11):2209–2220. https://doi.org/10.1021/acs.jpca.6b12125
Pu Z, Zhou H, Zheng Y, Huang W, Li X (2017) Enhanced methane combustion over Co3O4 catalysts prepared by a facile precipitation method: effect of aging time. Appl Surf Sci 410:14–21. https://doi.org/10.1016/j.apsusc.2017.02.186
Wilburn MS, Epling WS (2017) Sulfur deactivation and regeneration of mono- and bimetallic Pd-Pt methane oxidation catalysts. Appl Catal B Environ 206:589–598. https://doi.org/10.1016/j.apcatb.2017.01.050
García-Vázquez M, Wang K, González-Carballo JM, Brown D, Landon P, Tooze R, García-García FR (2020) Iron and chromium-based oxides for residual methane abatement under realistic conditions: a study on sulfur dioxide poisoning and steam-induced inhibition. Appl Catal B: Environ 277:119139. https://doi.org/10.1016/j.apcatb.2020.119139
Acknowledgements
Authors are grateful to the Centre for Nanoscience and Engineering (CeNSE), IISc for the XRD, XPS facilities and Department of Advanced Facilities for Microscopic and Macroscopic analysis (AFMM), IISc for providing TEM facility. GM thanks the Department of Science and Technology (DST), India for J.C. Bose fellowship (DST 1429). Dr. S.A. Singh thanks BITS-Pilani, Hyderabad campus for providing financial support by Research Initiation Grant (RIG-733) and Additional Competitive Research Grant (ACRG-906).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human and/or Animal Rights
There were no human or animal subjects involved in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S.A., Madras, G. & Sreedhar, I. Transition Metal (Ni, Cu and Fe) Substituted Co3O4 – ZrO2 Catalysts for Lean Methane Combustion. Top Catal 64, 243–255 (2021). https://doi.org/10.1007/s11244-020-01382-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01382-0