Abstract
Purpose
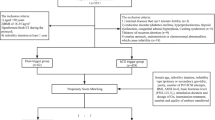
To compare morphokinetic parameters and quality of embryos derived from GnRH antagonist ICSI cycles triggered either with GnRH agonist or standard hCG between matched groups of patients.
Methods
Morphokinetic parameters of embryos derived from matched first GnRH antagonist ICSI cycles triggered by GnRH agonist or standard hCG between 2013 and 2016 were compared. Matching was performed for maternal age, peak estradiol levels, and number of oocytes retrieved. Outcome measures were: time to pronucleus fading (tPNf), cleavage timings (t2-t8), synchrony of the second and third cycles (S2 and S3), duration of the second and third cycle (CC2 and CC3), optimal cell cycle division parameters, and known implantation data (KID) scoring for embryo quality. Multivariate linear and logistic regression analyses were performed for confounding factors.
Results
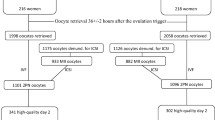
We analyzed 824 embryos from 84 GnRH agonist trigger cycles and 746 embryos from 84 matched hCG trigger cycles. Embryos derived from the cycles triggered with hCG triggering cleaved faster than those deriving from GnRH agonist trigger. The differences were significant throughout most stages of embryo development (t3-t6), and a shorter second cell cycle duration of the hCG trigger embryos was observed. There was no difference in synchrony of the second and third cell cycles and the optimal cell cycle division parameters between the two groups, but there was a higher percentage of embryos without multinucleation in the hCG trigger group (27.8% vs. 21.6%, p < 0.001).
Conclusion
The type of trigger in matched antagonist ICSI cycles was found to affect early embryo cleavage times but not embryo quality.
Similar content being viewed by others
References
Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–419.e5. https://doi.org/10.1016/j.fertnstert.2013.04.021.
Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–85. https://doi.org/10.1093/humrep/des079.
Rubio I, Galán A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102:1287–1294.e5. https://doi.org/10.1016/j.fertnstert.2014.07.738.
Baart EB, Macklon NS, Fauser BJ. Ovarian stimulation and embryo quality. Reprod BioMed Online. 2009;18:S45–50. https://doi.org/10.1016/S1472-6483(10)60448-8.
Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NGM, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–8. https://doi.org/10.1093/humrep/del484.
Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod BioMed Online. 2006;12:608–15. https://doi.org/10.1016/S1472-6483(10)61187-X.
Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: a systematic review and meta-analysis. PLoS One. 2017;12:e0175985. https://doi.org/10.1371/journal.pone.0175985.
Griesinger G, Kolibianakis EM, Papanikolaou EG, Diedrich K, Van Steirteghem A, Devroey P, et al. Triggering of final oocyte maturation with gonadotropin-releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen-thawed embryo replacement cycles. Fertil Steril. 2007;88:616–21. https://doi.org/10.1016/j.fertnstert.2006.12.006.
Hernández ER, Gómez-Palomares JL, Ricciarelli E. No room for cancellation, coasting, or ovarian hyperstimulation syndrome in oocyte donation cycles. Fertil Steril. 2009;91:1358–61. https://doi.org/10.1016/j.fertnstert.2008.03.077.
Itskovitz-Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndro. Hum Reprod. 2000;177:7–11. https://doi.org/10.1083/jcb.200611141.
Orvieto R. Can we eliminate severe ovarian hyperstimulation syndrome? Hum Reprod. 2005;20:320–2. https://doi.org/10.1093/humrep/deh613.
Humaidan P, Kol S, Papanikolaou EG. GnRH agonist for triggering of final oocyte maturation: Time for a change of practice? Hum Reprod Update. 2011;17:510–24. https://doi.org/10.1093/humupd/dmr008.
Itskovitz J, Boldes R, Levron J, Erlik Y, Kahana L, Brandes JM. Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil Steril. 1991;56:213–20.
Suda T, Balakier H, Powell W, Casper RF. Use of Gonadotropin-Releasing Hormone Agonist to Trigger Follicular Maturation for in Vitro Fertilization. J Clin Endocrinol Metab. 1990;71:918–22. https://doi.org/10.1210/jcem-71-4-918.
Humaiden P, Papanikolaou EG, Kyrou D, Alsbjerg B, Polyzos NP, Devroey P, et al. The luteal phase after GnRH-agonist triggering of ovulation: present and future perspectives. Reprod BioMed Online. 2012;24:134–41.
Kol S, Humaiden P. LH (as HCG) and FSH surges for final oocyte maturation: sometimes it takes two to tango? Reprod BioMed Online. 2010;21:590–2. https://doi.org/10.1016/j.rbmo.2010.06.031.
Muñoz M, Cruz M, Humaidan P, Garrido N, Pérez-Cano I, Meseguer M. The type of GnRH analogue used during controlled ovarian stimulation influences early embryo developmental kinetics: a time-lapse study. Eur J Obstet Gynecol Reprod Biol. 2013;168:167–72. https://doi.org/10.1016/j.ejogrb.2012.12.038.
Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum Reprod. 2000;15:2634–43.
Petersen BM, Boel M, Montag M, Gardner DK. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on Day 3. Hum Reprod. 2016;31:2231–44.
Pellicer A, Ruiz A, Castellvi RM, Calatayud C, Ruiz M, Tarin JJ, et al. Is the retrieval of high numbers of oocytes desirable in patients treated with gonadotrophin-releasing hormone analogues (GnRHa) and gonadotrophins? Hum Reprod. 1989;4:536–40. https://doi.org/10.1093/oxfordjournals.humrep.a136940.
van der Gaast MH, Eijkemans MJC, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod BioMed Online. 2006;13:476–80. https://doi.org/10.1016/S1472-6483(10)60633-5.
Rubio C, Mercader A, Alamá P, Lizán C, Rodrigo L, Labarta E, et al. Prospective cohort study in high responder oocyte donors using two hormonal stimulation protocols: impact on embryo aneuploidy and development. Hum Reprod. 2010;25:2290–7. https://doi.org/10.1093/humrep/deq174.
Gurbuz AS, Gode F, Uzman MS, Ince B, Kaya M, Ozcimen N, et al. GnRH agonist triggering affects the kinetics of embryo development: a comparative study. J Ovarian Res. 2016;9:22. https://doi.org/10.1186/s13048-016-0229-8.
Alyasin A, Mehdinejadiani S. GnRH Agonist trigger versus HCG trigger in GnRH antagonist in IVF/ICSI cycles: a review article. Int J Reprod Biomed. 2016;14:557–66.
Shapiro BS, Andersen CY. Major drawbacks and additional benefits of agonist trigger-not ovarian hyperstimulation syndrome related. Fertil Steril. 2015;103:874–8.
Humaidan P, Engmann L, Benadiva C. Luteal phase supplementation after gonadotropin-releasing hormone agonist trigger in fresh embryo transfer: the American versus European approaches. Fertil Steril. 2015;103:879–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oron, G., Sapir, O., Wertheimer, A. et al. A matched propensity score study of embryo morphokinetics following gonadotropin-releasing hormone agonist versus human chorionic gonadotropin trigger. J Assist Reprod Genet 37, 2777–2782 (2020). https://doi.org/10.1007/s10815-020-01953-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01953-w