Abstract
Background and purpose
Osteoarthritis (OA) impacts the quality of life in middle-aged and elderly people by inducing immobility. The severe inflammation in chondrocytes is reported to be related to the development and process of OA. The present study aims to investigate the protective effects of Apremilast on injured chondrocytes induced by interleukin-1α (IL-1α) and the underlying mechanism.
Methods
10 ng/mL IL-1α was used to induce the in vitro injured chondrocytes. QRT-PCR was used to evaluate the expression level of Sry-type high-mobility-group box 9 (SOX-9), collagen type II alpha-1 gene (COL2A1), Aggrecan (ACAN) and collagen type X alpha 1 chain (COL10A1). SiRNA technology was utilized to knock down the expression of SOX-9 in the chondrocytes. The expression of SOX-9 was determined by Western Blot assay and/or immunofluorescence assay. Western Blot was used to evaluate the expression level of phosphorylated cyclic AMP response element binding (CREB).
Results
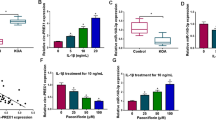
SOX9, Col2a1 and Acan were significantly up-regulated and Col10a1 was significantly down-regulated in the chondrocytes by Apremilast in a dose-dependent manner. IL-1α induced the injured chondrocytes by decreasing the expression of SOX9, Col2a1, Acan and increasing the expression of Col10a1, which were greatly reversed by Apremilast. By silencing SOX-9, the effects of Apremilast on SOX9 and marker genes were abolished. Phosphorylated CREB was up-regulated by Apremilast in a time-dependent manner. The up-regulated SOX-9 by Apremilast was reversed by the protein kinase A (PKA)/CREB pathway inhibitor H89.
Conclusion
Apremilast may protect chondrocytes from inflammation by up-regulating SOX9.








Similar content being viewed by others
References
Pelletier JP, et al. Immunological analysis of proteoglycan structural changes in the early stage of experimental osteoarthritic canine cartilage lesions. J Orthop Res. 1992;10(4):511–23.
Adams ME. Cartilage hypertrophy following canine anterior cruciate ligament transection differs among different areas of the joint. J Rheumatol. 1989;16(6):818–24.
Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227.
Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51(6):941–6.
Hayashi D, Roemer FW, Guermazi A. Imaging for osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):161–9.
Carames B, et al. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801.
Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8(2):333–45.
Taniguchi N, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106(4):1181–6.
Johnson EO, et al. Apoptosis in osteoarthritis: morphology, mechanisms, and potential means for therapeutic intervention. J Surg Orthop Adv. 2008;17(3):147–52.
Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21(11):1342–7.
Verdonk P, et al. Cyclodextrin polysulphates repress IL-1 and promote the accumulation of chondrocyte extracellular matrix. Osteoarthr Cart. 2005;13(10):887–95.
Bar Oz M, et al. Acetylation reduces SOX9 nuclear entry and ACAN gene transactivation in human chondrocytes. Aging Cell. 2016;15(3):499–508.
Otero M, et al. ELF3 modulates type II collagen gene (COL2A1) transcription in chondrocytes by inhibiting SOX9-CBP/p300-driven histone acetyltransferase activity. Connect Tissue Res. 2017;58(1):15–26.
Li C, et al. MicroRNA-140 suppresses human chondrocytes hypertrophy by targeting SMAD1 and controlling the bone morphogenetic protein pathway in osteoarthritis. Am J Med Sci. 2018;355(5):477–87.
Keating GM. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2017;77(4):459–72.
Bi W, et al. Sox9 is required for cartilage formation. Nat Genet. 1999;22(1):85–9.
Stokes DG, et al. Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. Biochem J. 2001;360(Pt 2):461–70.
Cao L, et al. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials. 2011;32(16):3910–20.
Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–8.
Kolettas E, et al. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of SOX-9 transcription factor. Rheumatol (Oxf). 2001;40(10):1146–56.
Perera PM, et al. Mechanical signals control SOX-9, VEGF, and c-Myc expression and cell proliferation during inflammation via integrin-linked kinase, B-Raf, and ERK1/2-dependent signaling in articular chondrocytes. Arthritis Res Ther. 2010;12(3):R106.
Lee HJ, et al. Membrane-free stem cell components inhibit interleukin-1alpha-stimulated inflammation and cartilage degradation in vitro and in vivo: a rat model of osteoarthritis. Int J Mol Sci. 2019;20(19):4869.
Deffaud J, et al. Modulatory effect of rhein on IL-1alpha-induced responses in human chondrocytes: a comparative study between antibody microarrays and specific ELISAs. Biorheology. 2008;45(3–4):439–55.
Ikegami D, et al. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development. 2011;138(8):1507–19.
Akiyama H, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–28.
Aigner T, Stove J. Collagens–major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003;55(12):1569–93.
van den Berg WB. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthr Cart. 2011;19(4):338–41.
Hu G, Codina M, Fisher S. Multiple enhancers associated with ACAN suggest highly redundant transcriptional regulation in cartilage. Matrix Biol. 2012;31(6):328–37.
Diederichs S, et al. Chondral differentiation of induced pluripotent stem cells without progression into the endochondral pathway. Front Cell Dev Biol. 2019;7:270.
Huang H, et al. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44(5):420–7.
Siddappa R, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci USA. 2008;105(20):7281–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Huang, X. & Yuan, Y. Anti-inflammatory capacity of Apremilast in human chondrocytes is dependent on SOX-9. Inflamm. Res. 69, 1123–1132 (2020). https://doi.org/10.1007/s00011-020-01392-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01392-4