Abstract
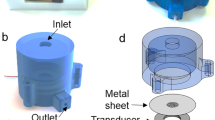
3D bioprinting is one of the rapidly evolving fields of tissue engineering where microengineering meets cells biology within an unprecedented precision to construct tissue structures of various forms with complexity. However, enabling simultaneous printing of heterogeneous biomaterial along with scaffold components through the currently available printers is still considered as a major challenge due to the lack of instrumentation. Flow control is one of the major issues associated with the process. In this aspect, a microfluidic nozzle head equipped with two shape-memory alloy (SMA) actuators was proposed and integrated with a commercially available 3D printer to assist the biomaterial printing in a more systematic manner. The SMA actuator restrains the amount of flows for fabricating the desired scaffold components. Experimental results illustrated that the use of SMA actuator ensued a rapid and precise flow control of biomaterial and can further facilitate to maintain the width of any printed structures. As a proof of concept for the profound biomedical applications with the present manufacturing configuration, a 3D printed hydrogel platform was fabricated with demonstrated characters for later cell seeding after the printing further opens a new chapter in terms of biomaterial printing.






Similar content being viewed by others
References
Hopkinson, N., Hague, R. & Dickens, P. Rapid manufacturing: an industrial revolution for the digital age. (John Wiley & Sons, 2006).
Wong, K.V. & Hernandez, A. A review of additive manufacturing. ISRN Mech. Eng., 2012 208760 (2012).
Huang, S.H., Liu, P., Mokasdar, A. & Hou, L. Additive manufacturing and its societal impact: a literature review. Int. J. Adv. Manuf. Tech., 67 1191–1203 (2013).
Vaezi, M., Seitz, H. & Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Tech., 67 1721–1754 (2013).
Ozbolat, I.T. Special issue on three-dimensional bioprinting. J. Nanotechnol. Eng. Med., 6 020301 (2015).
Mironov, V., Trusk, T., Kasyanov, V., Little, S., Swaja, R. & Markwald, R. Biofabrication: a 21st century manufacturing paradigm. Biofabrication, 1 022001 (2009).
Murphy, S.V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol., 32 773–785 (2014).
Zhu, W., Ma, X., Gou, M., Mei, D., Zhang, K. & Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol., 40 103–112 (2016).
Huh, D., Hamilton, G.A. & Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol., 21 745–754 (2011).
Crowley, C., Birchall, M. & Seifalian, A.M. Trachea transplantation: from laboratory to patient. J. Tissue Eng. Regener. Med., 9 357–367 (2015).
Mironov, V., Visconti, R.P., Kasyanov, V., Forgacs, G., Drake, C.J. & Markwald, R.R. Organ printing: tissue spheroids as building blocks. Biomaterials, 30 2164–2174 (2009).
Ovsianikov, A., Gruene, M., Pflaum, M., Koch, L., Maiorana, F., Wilhelmi, M., Haverich, A. & Chichkov, B. Laser printing of cells into 3D scaffolds. Biofabrication, 2 014104 (2010).
Ilkhanizadeh, S., Teixeira, A.I. & Hermanson, O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials, 28 3936–3943 (2007).
Kirchmajer, D.M. & Gorkin III, R. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J Mater. Chem. B, 3 4105–4117 (2015).
Sun, J., Ng, J.H., Fuh, Y.H., Wong, Y.S., Loh, H.T. & Xu, Q. Comparison of micro-dispensing performance between micro-valve and piezoelectric printhead. Microsyst. Technol., 15 1437–1448 (2009).
Dai, G. & Lee, V. Three-dimensional bioprinting and tissue fabrication: prospects for drug discovery and regenerative medicine. Adv. Health Care Technol, 1 23–35 (2015).
Ozbolat, I. T. & Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials, 76 321–343 (2016).
Hölzl, K., Lin, S., Tytgat, L., Van Vlierberghe, S., Gu, L. & Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication, 8 032002 (2016).
Blaeser, A. Campos, D.F.D., Puster, U., Richtering, W., Stevens, M.M. & Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthcare Mater., 5 326–333 (2016).
Chen, C.-Y., Chen, C.-Y., Lin, C.-Y. & Hu, Y.-T. Magnetically actuated artificial cilia for optimum mixing performance in microfluidics. Lab Chip, 13 2834–2839 (2013).
Panigrahi, B. & Chen, C.-Y. Microfluidic retention of progressively motile zebrafish sperms. Lab Chip, 19 4033–4042 (2019).
Atencia, J. & Beebe, D.J. Controlled microfluidic interfaces. Nature, 437 648–655 (2005).
Jacobson, S.C., Ermakov, S.V. & Ramsey, J.M. Minimizing the number of voltage sources and fluid reservoirs for electrokinetic valving in microfluidic devices. Anal. Chem., 71 3273–3276 (1999).
Voldman, J., Gray, M.L. & Schmidt, M.A. An integrated liquid mixer/valve. J. Microelectromech. Syst., 9 295–302 (2000).
Sundararajan, N., Kim, D.S. & Berlin, A.A. Microfluidic operations using deformable polymer membranes fabricated by single layer soft lithography. Lab Chip, 5 350–354 (2005).
Irimia, D., Liu, S.-Y., Tharp, W.G., Samadani, A., Toner, M. & Poznansky, M.C. Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab Chip, 6 191–198 (2006).
Li, N.Z., Hsu, C.H. & Folch, A. Parallel mixing of photolithographically defined nanoliter volumes using elastomeric microvalve arrays. Electrophoresis, 26 3758–3764 (2005).
Baek, J.Y., Park, J.Y., Ju, J.I., Lee, T.S. & Lee, S.H. A pneumatically controllable flexible and polymeric microfluidic valve fabricated via in situ development. J. Micromech. Microeng., 15 1015–1020 (2005).
Gu, W., Chen, H. & Tung, Y.-C. Multiplexed hydraulic valve actuation using ionic liquid filled soft channels and Braille displays. Appl. Phys. Lett., 90 033505 (2007).
Weibel, D.B., Siegel, A.C., Lee, A., George, A.H. & Whitesides, G.M. Pumping fluids in microfluidic systems using the elastic deformation of poly (dimethylsiloxane). Lab Chip, 7 1832–1836 (2007).
Kaigala, G.V., Hoang, V.N. & Backhouse, C.J. Electrically controlled microvalves to integrate microchip polymerase chain reaction and capillary electrophoresis. Lab Chip, 8 1071–1078 (2008).
Yang, B.Z. & Lin, Q. A latchable microvalve using phase change of paraffin wax. Sens. Actuators, A, 134 194–200 (2007).
Snyder, J., Son, A.R., Hamid, Q., Wu, H. & Sun, W. Hetero-cellular prototyping by synchronized multimaterial bioprinting for rotary cell culture system. Biofabrication, 8 015002 (2016).
Bsoul, A., Pan, S., Cretu, E., Stoeber, B. & Walus, K. Design, microfabrication, and characterization of a moulded PDMS/SU-8 inkjet dispenser for a Lab-on-a-Printer platform technology with disposable microfluidic chip. Lab Chip, 16 3351–3361 (2016).
Benda, P., Lightbody, J., Sato, G., Levine, L. & Sweet, W. Differentiated rat glial cell strain in tissue culture. Science, 161 370–371 (1968).
Wiche, G. & Cole, R.D. Reversible in vitro polymerization of tubulin from a cultured cell line (rat glial cell clone C6). Proc. Natl. Acad. Sci. U.S.A., 73 1227–1231 (1976).
Wu, H.M. Fabrication of nitinol materials and components. Mater. Sci. Forum, 394 285–292 (2002).
Mani, K., Chien, T.-C.C., Panigrahi, B. & Chen, C.-Y. Manipulation of zebrafish’s orientation using artificial cilia in a microchannel with actively adaptive wall design. Sci. Rep., 6 36385 (2016).
Lu, Y.-H., Mani, K., Panigrahi, B., Hajari, S. & Chen, C.-Y. A shape memory alloy-based miniaturized actuator for catheter interventions. Cardiovasc. Eng. Technol., 9 405–413 (2018).
Mc Caffrey, C., Umedachi, T., Jiang, W., Sasatani, T., Narusue, Y., Niiyama, R. & Kawahara, Y. Continuum robotic caterpillar with wirelessly powered shape memory alloy actuators. Soft Robotics DOI https://doi.org/10.1089/soro.2019.0090 (2020)
Asamura, K. & Nagasawa, S. MEMS fabrication of compliant sheet for micro hexapod robots. Jpn. J. Appl. Phys. 59, DOI https://doi.org/10.35848/1347-4065/ab7439 (2020).
References
He, Y., Yang, F.F., Zhao, H.M., Gao, Q., Xia, B. & Fu, J.Z. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 6, 29977 (2016).
Wong, K.H., Chan, J.M., Kamm, R.D. & Tien, J. Microfluidic models of vascular functions. Annu. Rev. Biomed. Eng. 14, 205–230 (2012).
Dewey, C., Bussolari, S., Gimbrone, M. & Davies, P. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 103, 177–185 (1981).
Blaeser, A., Campos, D.F.D, Puster, U., Richtering, W., Stevens, M.M. & Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthcare Mater. 5, 326–333 (2016).
Chang, R., Nam, J. & Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng., Part A 14, 41–48 (2008).
Acknowledgements
This study was supported through the Ministry of Science and Technology of Taiwan under Contract No. MOST 108-2221-E-006-22 1-MY4 (to Chia-Yuan Chen). This work would not be possible without the facility provided by Center for Micro/Nano Science and Technology, National Cheng Kung University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mani, K., Lin, WC., Wang, CF. et al. A Multi-Inlet Microfluidic Nozzle Head with Shape Memory Alloy-Based Switching for Biomaterial Printing with Precise Flow Control. BioChip J 14, 340–348 (2020). https://doi.org/10.1007/s13206-020-4402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-020-4402-1