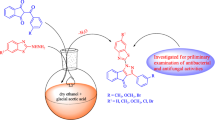
A series of new 2-(4/3-substitutedbenzyl)thieno[2,3-d]pyrimidin-4(3H)-ones (2a-f) and 2-(4/3-substituted benzyl)-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one derivatives (3a-f) were synthesized for the first time by the reaction of corresponding 2-aminothiophene-3-carboxamide derivatives and appropriate iminoester hydrochlorides under suitable conditions. Using microdilution method, the antibacterial and antifungal activities of the synthesized compounds were tested against selected strains of Gram positive and Gram negative bacteria (Staphylococcus aureus, Enterecoccus faecalis, Escherichia coli and Pseudomonas aeruginosa) and yeast-like fungi (Candida albicans, C. krusei and C. parapsilosis). In these tests, compounds 3a-f showed higher antifungal activity than fluconazole against Candida fungus species. 2-Substituted thieno-[2,3-d]pyrimidin-4(3H)-ones (2a-f) showed better antibacterial activity than 2-substituted 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one derivatives (3a-f).



Similar content being viewed by others
References
T. Eicher and S. Hauptmann, The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications, 2nd Edition, Wiley-VCH Verlag GmbH (2005), pp. 1 – 4.
A. J. Folkes, K. Ahmadi, W. K. Alderton, et al., J. Med. Chem., 51(18), 5522 – 5532 (2008).
F. E. M. El-Baih, H. A. S. Al-Blowy, H. M. Al-Hazimi, Molecules, 11(7), 498 – 513 (2006).
R. V. Chambhare, B. G. Khadse, A. S. Bobde, et al., Eur. J. Med. Chem., 38(1), 89 – 100 (2003).
N. T. Pokhodylo, O. Y. Shyyka, R. D. Savka, et al., Phosphorus, Sulfur, Silicon, 185, 2092 – 2100 (2010).
M. R. Bhadane, J. N. N. Sharath Chandra, and L. V. G. Nargund, Der Pharma Chemica, 3(4), 238 – 244 (2011).
P. Rashmi, L. V. G. Nargund, K. Hazra, et al., Arch. Pharm. Chem. Life Sci., 344, 459 – 465 (2011).
V. K. Akbari, P. D. Patel, and K. C. Patel, Int. J. Chem. Tech. Res., 5(1), 142 – 155 (2013)
X. Ji, T. Peng, X. Zhang, et al., Bioorg. Med. Chem., 22(7), 2366 – 2378 (2014).
J. A. S. Mulla and M. I. A. Khazi, Med. Chem. Res., 23(6), 3235 – 3243(2014).
S. Saxena, G. Samala, J. Renuka, et al., Bioorg. Med. Chem., 23(7), 1402 – 1412 (2015).
J. W. De Schutter, J. Park, C. Y. Leung, et al., J. Med. Chem., 57(13), 5764 – 5776 (2014).
X.-F. Wu, S. Oschatz, M. Sharif, et al., Tetrahedron, 70(1), 23 – 29 (2014).
X. Yang, G. Cheng, J. Shen, et al., Org. Chem. Front., 2, 366 – 368 (2015).
M. R. Prasad, A. R. Rao, P. S. Rao, et al., J. Chem. Res. (S), 5 (2002); J. Chem. Res. (M), 0149 (2002).
S. Hesse, E. Perspicace, and G. Kirsch, Tetrahedron Lett., 48(30), 5261 – 5264 (2007).
V. Goudar, P. Rashmi, U. Shantharam, et al., J. Chem. Pharm. Res., 4(6), 3100 – 3106 (2012).
L. Ouyang, L. Zhang, J. Liu, et al., J. Med. Chem., 60, 9990 – 10012 (2017).
A. Kumar Verma, A. Martin, R. Kant, et al., J. Pharm. Res., 8(11), 1677 – 1681 (2014).
A. S. Abd El-All, S. M. Sh. Atta, H. M. F. Roaiah, et al., Arch. Pharm. Chem. Life Sci., 349, 202–210 (2016).
A. Pinner, Dieimidoether und ihre Derivate, 1. Auflage, Oppenheim, Berlin (1892).
E. Menteşe, H. Bektaş, S. Ülker, et al., J. Enzym. Inhib. Med. Chem., 29(1), 64 – 68 (2014).
National Committee for Clinical Laboratory Standarts (NCCLS). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 8th ed., M 07-A8. Clinical and Laboratory Standards Institute, Wayne, PA (2008).
National Committee for Clinical Laboratory Standarts (NCCLS). Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 3rd ed., M 27-A3. Clinical and Laboratory Standards Institute, Wayne, PA (2008).
M. R. Prasad and D. P. Kishore, Chem. Pharm. Bull., 55(5), 776 – 780 (2007).
C. J. Shishoo, M. B. Devani, S. Ananthan, et al., Indian J. Chem., Sect. B, 28B(12), 1039 – 1045 (1989).
Acknowledgements
This study was supported by the Scientific Research Project Coordination Unit of Karadeniz Technical University. Project number: FBA-2016-5461.
We would like to thank Assist. Prof. Dr. Hasan SAĞLAMEL for the English review. We would like to thank Statistician Tuðba GÜVEN
KURT, KTÜ Farabi Hospital, Statistical Unit for the statistical data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kahveci, B., Doğan, İ.S., Menteşe, E. et al. Synthesis and Antibacterial and Antifungal Activity of New Thieno[2,3-d]Pyrimidin-4(3H)-One Derivatives. Pharm Chem J 54, 647–653 (2020). https://doi.org/10.1007/s11094-020-02252-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02252-5