Abstract
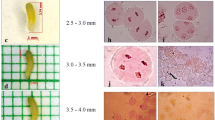
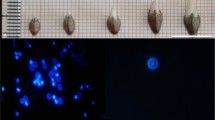
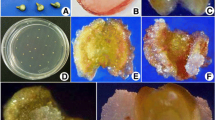
A protocol for production of haploids from two heterozygous male sterile lines of Tagetes erecta using un-pollinated ovules as explant was standardized. Various factors affecting the in vitro gynogenic response viz., growth regulators, ovule developmental stage, basal media, sucrose concentration, photoperiod and cold shock duration were assessed in Tagetes erecta. Direct induction of parthenogenic embryos occurred in cultivar ‘Local Orange’ when the un-pollinated ovules were cultured in EMS (MS medium enriched with coconut water, AgNO3, PVP etc.) medium supplemented with 2 mg l−1 BAP and 0.5 mg l−1 NAA or 2 mg l−1 BAP along with 2 mg l−1 2,4-D. The developmental stage of the flower buds was vital in embryo induction from the excised ovules. The ovules collected from half-open flower buds were more responsive to gynogenesis as compared to ovules collected from un-open and fully open flower buds. EMS basal medium was found best for gynogenesis over the other commercially available basal media. Cold pre-treatment of flower buds at 4 ºC had no stimulatory effect and negatively affected the gynogenesis. The dark culture condition was imperative for direct parthenogenic embryo induction than 16 h light duration. The ploidy levels of 17 regenerants were determined by cytological studies, revealed 47.06% as diploids, 41.18% as haploids and 11.76% as mixoploids. These results were reconfirmed with flow cytometry analysis. The determination of ploidy level by counting the number of chloroplasts in stomatal guard cells of marigold was also established for rapid screening of haploids. The resultant haploids were successfully diploidised and are being utilized in the hybrid breeding programme at our institute. The standardized protocol for doubled haploids (DHs) production by un-pollinated ovule culture paved the way for Tagetes F1 hybrid breeding.
Key message
This is the first successful report of in vitro gynogenesis in Tagetes spp for production of doubled haploids (DHs). The protocol for obtaining high frequency parthenogenic embryo induction from unpollinated ovules was standardized and validated for rapid production of homozygous lines.





Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BAP:
-
Benzylamino purine
- EMS:
-
Enriched Murashige and Skoog medium
- NAA:
-
1-Naphthalene-acetic acid
- SO:
-
Seracole Orange
- LO:
-
Local Orange
- PMS:
-
Petalous male sterile
References
Ahmadi B, Ebrahimzadeh H (2020) In vitro androgenesis: spontaneous vs. artificial genome doubling and characterization of regenerants. Plant Cell Rep 39:299–316
Alan AR, Toprak FC, Kaska A (2016) Production and evaluation of gynogenic leek (Allium ampeloprasum L.) plants. Plant Cell Tiss Org 125:249–259
Anandhan S, Ashwini A, Chavan GJ, Mote SR, Poonam V, Lawande KE (2014) Variation in gynogenic potential for haploid induction in Indian short day onions. Indian J Genet 74(4):526–528
Anonymous (2014) Indian Horticulture data base. https://nhb.gov.in
Anonymous (2016) https://www.eximpulse.com/import-product-Marigold-Seeds-country.htm
Bhagyalakshmi N (1999) Factors influencing direct shoot regeneration from ovary explants of saffron. Plant Cell Tiss Org 58:205–211
Bhat JG, Murthy HN (2007) Factors affecting in vitro gynogenic haploid production in Niger (Guizotia abyssinica (L. f) Cass.). Plant Growth Regul 52:241–248
Bhatia R, Dey SS, Shritika S, Sharma K, Sharma VK, Parkash C, Kumar R (2016) Optimizing protocol for efficient microspore embryogenesis and doubled haploid development in different maturity groups of cauliflower (B. oleracea var. botrytis L.) in India. Euphytica 212:439–454
Bhojwani SS, Dantu PK (2013) Gynogenesis. Plant tissue culture: an introductory text. Springer, India, pp 113–118
Bohanec B (2009) Doubled haploids via gynogenesis. In: Touraev A, Forster B, Jain M (eds) Advances in haploid production in higher plants. Springer, Heidelberg, pp 35–46
Castillo AM, Cistue L (1993) Production of gynogenic haploids of Hordeum vulgare L. Plant Cell Rep 12:139–143
Chen JF, Cui L, Malik AA, Mbira KG (2011) In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tiss Org 104(3):311–319
Chu CC, Wang CC, Sun CS, Hsu C, Yin KC, Chu CY, Bi FI (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci Sin 18:659–668
Davojan EI (1985) Obtaining rice haploids from unpollinated ovaries culture technique in vitro conditions. Doklady Vashnil 3:15–16
Eyster WH (1941) The induction of fertility in genetically self-sterile plants. Science 94:144–145
Ferrant V, Bouharmont J (1994) Origin of gynogenetic embryos of Beta vulgaris L. Sex Plant Reprod 7:12–16
Gamborg OL, Miller RA, Rancillac M (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gelebart P, San LH (1987) Production of haploid plants of sunflower (Helianthus annuus L.) by in vitro culture of non-fertilized ovaries and ovules. Agronomie 7:81–86
Gémes-Juhász A, Balogh P, Ferenczy A, Kristóf Z (2002) Effect of optimal stage of female gametophyte and heat treatment on in vitro gynogenesis induction in cucumber (Cucumis sativus L.). Plant Cell Rep 21:105–111
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Kumar RK (2017) Studies on plant regeneration in marigold (Tagetes spp.) through in vitro culture of male and female gametophytes. PhD Thesis, Indian Agricultural Research Institute, New Delhi, India
Kumar RK, Singh KP, Raju DVS, Panwar S, Bhatia R, Jain PK, Vinod (2017) Standardization of rapid multiplication protocol in petaloid male sterile lines of African marigold (Tagetes erecta) through in vitro culture. Indian J Agr Sci 87(10):1295–1302
Kumar KR, Singh KP, Bhatia R, Raju DVS, Panwar S (2019) Optimising protocol for successful development of haploids in marigold (Tagetes spp.) through in vitro androgenesis. Plant Cell Tiss Org 138:11–28
Lakshmi Sita L (1996) Gynogenic haploids in vitro. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol 4. Kluwer, Dordrecht, pp 175–193
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z Pflanzenphysiol 105:427–434
MartÍnez LE, Aguero CB, Lopez ME, Galmarini CR, (2000) Improvement of in vitro gynogenesis induction in onion (Allium cepa L.) using polyamines. Plant Sci 156:221–226
Metwally EI, Moustafa SA, El-Sawy BI, Haroun SA, Shalaby TA (1998) Production of haploid plants from in vitro culture of unpollinated ovules of Cucurbita pepo. Plant Cell Tiss Org 52:117–121
Michalik B, Adamus A, Nowak E (2000) Gynogenesis in polish onion cultivars. J Plant Physiol 156:211–216
Miler N, Muszczyk P (2015) Regeneration of callus and shoots from the ovules and ovaries of chrysanthemum in vitro. Acta Hortic 1083:58
Miyoshi K, Asakura N (1996) Callus induction, regeneration of haploid plants and chromosome doubling in ovule cultures of pot gerbera (Gerbera jamesonii). Plant Cell Rep 16:1–5
Mukhambetzhanov SK (1997) Culture of nonfertilized female gametophytes in vitro. Plant Cell Tiss Org 48:111–119
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Puddephat IJ, Robinson HT, Smith BM (1999) Influence of stock plant pretreatment on gynogenic embryo induction from buds of onion. Plant Cell Tiss Org 57:145–148
San Noeum LH, Gelebart P (1986) Production of gynogenic haploids. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol. 3: Academic, New York, pp 305–322
Sato S, Katoh N, Yoshida H, Iwail S, Hagimori M (2000) Production of doubled haploid plants of carnation (Dianthus caryophyllus L.) by pseudo fertilized ovule culture. Sci Hort 83:301–310
Schum A, Mattiesch l, Timmann EM, Hofmann (1993) Regeneration of diploids via gynogenesis in Allium porrum L. Gartenbauwissenschaft 58:227–232
Singh B, Swarup V (1973) Inheritance of qualitative characters in African marigold. Indian J Genet Pl Br 33(3):373–377
Sirikanya C, Mai C (2005) Inheritance of marigold flower characteristics. https://agris.fao.org/agris-search/search.do?recordID=TH2005001364
Sitbon M (1981) Production of haploid Gerbera jamesonii plants by in vitro culture of unfertilized ovules. Agronomie 1(9):807–812
Thomas DT, Bhatnagar AK, Razdan MK, Bhojwani SS (1999) A reproducible protocol for the production of gynogenic haploids of mulberry, Morus alba L. Euphytica 110:169–173
Tosca A, Arcara L, Frangi P (1999) Effect of genotyping and season of gynogenesis efficiency in gerbera. Plant Cell Tiss Org 59:7–80
Wang H, Dong B, Jiang J, Fang W, Guan Z, Liao Y, Chen S, Chen F (2014) Characterization of in vitro haploid and doubled haploid Chrysanthemum morifolium plants via unfertilized ovule culture for phenotypical traits and DNA methylation pattern. Front Plant Sci 5:1–10
Winner B (2005) Marigold hybrid 50011. United Sates patent No. U.S. 6,894,208 B2. Washington, DC: U.S.Patent and Trademark Office
Wu BJ, Chen KC (1982) Cytological and embryological studies on haploid plant production from cultured unpollinated ovaries of Nicotiana tabacum L. Acta Bot Sin 24:125–129
Yang HY, Yan H, Zhou C (1990) In vitro production of haploids in Helianthus. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry; legumes and oilseed crops I, vol 10. Springer. Berlin, Heidelberg, pp 472–484
Zhang P, Zeng L, Su Y, Gong X, Wang X (2011) Karyotype studies on Tagetes erecta L. and Tagetes patula L. Afr J Biotechnol 10 (72): 16138–16144
Ziv M, Kahana O (1988) The role of peanut (Araschis hypogaea) ovular tissue in the photo morphogenetic responce of the embryo. Plant Sci 25:159–164
Acknowledgements
Authors sincerely acknowledge the ICAR-Indian Agricultural Research Institute, New Delhi for providing the research facilities and Dr. YSR Horticultural University, Andhra Pradesh for grant of deputation to the first author to carrying out this research work.
Author information
Authors and Affiliations
Contributions
KRK: He is responsible for conducting all the tissue culture related experiments, characterizing the ovule derived regenerants and writing manuscript. KPS: He has planned the experiments and provided the basic research facility for conducting these experiments. RB: She has planned the experiments, assisted in conducting the ploidy analysis and correcting the manuscript. DVSR: He has assisted in planning and data analysis of this study. SP: She has contributed in collecting, raising and maintenance of the marigold cultivars and ovule derived regenerants.
Corresponding author
Ethics declarations
Conflict of interest
The consent of all the authors for submission of this manuscript has been taken. There is no conflict of interest among the authors of this manuscript.
Additional information
Communicated by Maria Antonietta Germanà.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, K.R., Singh, K.P., Raju, D.V.S. et al. Maternal haploid induction in African marigold (Tagetes erecta L.) through in vitro culture of un-fertilized ovules. Plant Cell Tiss Organ Cult 143, 549–564 (2020). https://doi.org/10.1007/s11240-020-01940-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01940-0