Abstract
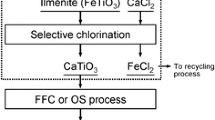
The ilmenite-chloride process has used for the production of TiCl4 from the ilmenite (FeTiO3) ore, which produces cyclone dust containing mostly iron chloride and includes a range of metal chlorides. The utilization of iron values present in waste chlorides of cyclone dust is becoming a crucial issue to make this process competitive. The current work demonstrates a beneficial process that can selectively separate iron values from the chloride residue. Using CaCO3 as a precipitating agent, the iron component was selectively isolated from the aqueous solution of the chloride residues. The selective extraction of iron was carried out at a wide range of concentrations, and the yield of iron species was over 95%. The precipitate is in the form of Fe(OH)3, which converts to Fe2O3 when annealed in air. In the next step, the remaining metal impurities were removed as solid precipitates through the pH tuning with CaO. Finally, CaCl2 and CaCO3 were obtained by adding CO2 to the residual solution. This study provides a method of treating cyclone residues to recover CaCl2 as well as Fe(OH)3, which represents significant progress towards the utilization of iron-rich wastes.







Similar content being viewed by others
References
Sibum H, Güther V, Roidl O, Habashi F, Wolf H-U (2000) Ullmann's encyclopedia of industrial chemistry, Edition: electronic release, Chapter: titanium, titanium alloys, and titanium compounds. Wiley-VCH, Weinheim, pp 1–35
Wensheng Z, Zhaowu Z, Chu Y-C (2011) A literature review of titanium metallurgical processes. Hydrometallurgy 108:177–188. https://doi.org/10.1016/j.hydromet.2011.04.005
Mehdilo A, Irannajad M (2012) Effects of mineralogical and textural characteristics of ilmenite concentrate on synthetic rutile production. Arab J Geosci 6:3865–3876. https://doi.org/10.1007/s12517-012-0647-x
Yuan Z, Wang X, Xu C, Li W, Kwauk M (2006) A new process for comprehensive utilization of complex titania ore. Miner Eng 19:975–978. https://doi.org/10.1016/j.mineng.2005.10.002
Fu X, Wang Y, Xiong L, Wei F (2009) Enhancement of the low temperature chlorination of ilmenite with CCl4 by adding Cl2. J Alloys Compd 486:365–370. https://doi.org/10.1016/j.jallcom.2009.06.149
Perkins E-C, Lang R-S, Dolezal H (1961) Chlorination of an Idaho ilmenite. [Washington, D.C.]: U.S. Dept. of the Interior, Bureau of Mines.
Joseph P-T, Mathew P-M (1969) A new method of processing ilmenite for titanium compounds. J Chem Soc D. https://doi.org/10.1039/C29690000374
Bordbar H, Yousefi A-A, Abedini H (2017) Production of titanium tetrachloride (TiCl4) from titanium ores: A review. Polyolefins J 4:149–173. https://doi.org/10.22063/poj.2017.1453
Raman R, Aarti K, Deepika K-S, Ranjit P, Ranganathan S (2019) Carbothermic reduction of iron oxide waste generated during the processing of ilmenite. Trans Indian Inst Met 72:11–16. https://doi.org/10.1007/s12666-018-1451-4
Das G-K, Pranolo Y, Zhu Z, Cheng C-Y (2013) Leaching of ilmenite ores by acidic chloride solutions. Hydrometallurgy 133:94–99. https://doi.org/10.1016/j.hydromet.2012.12.006
Kang J, Okabe T-H (2013) Removal of iron from titanium ore through selective chlorination using magnesium chloride. Mater Trans 54:1444–1453. https://doi.org/10.2320/matertrans.M-M2013810
Ward J, Bailey S, Avraamides J (1999) The use of ethylene diammonium chloride as an aeration catalyst in the removal of metallic iron from reduced ilmenite. Hydrometallurgy 53:215–232. https://doi.org/10.1016/S0304-386X(99)00046-8
Jha M-K, Kumar V, Singh R-J (2002) Solvent extraction of zinc from the chloride solutions. Solvent Extr Ion Exch 20:389–405. https://doi.org/10.1081/SEI-120004812
Niecko J (1987) Recovery of ferrous sulfate and sulfuric acid from spent pickle liquor of the steel industry. Conserv Recycl 10:309–314. https://doi.org/10.1016/0361-3658(87)90061-0
Lanyon M-R, Lwin T, Merritt R-R (1999) The dissolution of iron in the hydrochloric acid leach of an ilmenite concentrate. Hydrometallurgy 51:299–323. https://doi.org/10.1016/S0304-386X(98)00083-8
Jonglertjunya W, Rubcumintara T (2012) Titanium and iron dissolutions from ilmenite by acid leaching and microbiological oxidation techniques. Asia-Pac J Chem Eng 8:323–330. https://doi.org/10.1002/apj.1663
Pereira E-B, Suliman A-L, Tanabe E-H, Bertuol D-A (2018) Recovery of indium from liquid crystal displays of discarded mobile phones using solvent extraction. Miner Eng 119:67–72. https://doi.org/10.1016/j.mineng.2018.01.022
Hamza M-F, Roux J-C, Guibal E (2019) Metal valorization from the waste produced in the manufacturing of Co/Mo catalysts: leaching and selective precipitation. J Mater Cycles Waste Manag 21:525–538. https://doi.org/10.1007/s10163-018-0811-9
Busolic D, Parada F, Parra R, Sanchez M, Palacios J, Hino M (2011) Recovery of iron from copper flash smelting slags. Miner Process Extr Metall 120:32–36. https://doi.org/10.1179/037195510X12772935654945
Kumar V, Sahu S-K, Pandey B-D (2010) Prospects for solvent extraction processes in the Indian context for the recovery of base metals. A review. Hydrometallurgy 103:45–53. https://doi.org/10.1016/j.hydromet.2010.02.016
Xiang W, Liang S, Zhou Z, Qin W, Fei W (2017) Lithium recovery from salt lake brine by counter-current extraction using tributyl phosphate/FeCl3 in methyl isobutyl ketone. Hydrometallurgy 171:27–32. https://doi.org/10.1016/j.hydromet.2017.04.007
Hu B, Nakahiro Y, Wakamatsu T (1993) The effect of organic solvents on the recovery of fine mineral particles by liquid-liquid extraction. Miner Eng 6:731–742. https://doi.org/10.1016/0892-6875(93)90004-7
Zhang G, Chen D, Wei G, Zhao H, Wang L, Qi T, Meng F, Meng L (2015) Extraction of iron (III) from chloride leaching liquor with high acidity using tri-n-butyl phosphate and synergistic extraction combined with methyl isobutyl ketone. Sep Purif Technol 150:132–138. https://doi.org/10.1016/j.seppur.2015.07.001
Barrak H, Ahmedi R, Chevallier P, M’nif A, Laroche G, Hamzaoui A-H (2019) Highly efficient extraction of rare earth elements and others ions from green phosphoric acid medium using TMSEDTA@GO@Fe3O4 core-shell. Sep Purif Technol 222:145–151. https://doi.org/10.1016/j.seppur.2019.04.016
Amer S, Takahashi J-M, Luis A (1995) The recovery of zinc from the leach liquors of the CENIM-LNETI process by solvent extraction with di (2-ethylhexyl) phosphoric acid. Hydrometallurgy 37:323–337. https://doi.org/10.1016/0304-386X(94)00040-A
Tavakoli M-R, Dreisinger D-B (2013) Separation of vanadium from iron by solvent extraction using acidic and neutral organophosphorus extractants. Hydrometallurgy 141:17–23. https://doi.org/10.1016/j.hydromet.2013.10.008
Jackson E (1998) The solvent extraction behaviour of platinum (II) with P-50 oxime in aqueous chloride solutions. Miner Eng 11:651–656. https://doi.org/10.1016/S0892-6875(98)00049-1
Roosendael S-V, Roosen J, Banerjee D, Binnemans K (2019) Selective recovery of germanium from iron-rich solutions using a supported ionic liquid phase (SILP). Sep Purif Technol 221:83–92. https://doi.org/10.1016/j.seppur.2019.03.068
Mishra R-K, Rout P-C, Sarangi K, Nathsarma K-C (2011) Solvent extraction of Fe(III) from the chloride leach liquor of low grade iron ore tailings using Aliquat 336. Hydrometallurgy 108:93–99. https://doi.org/10.1016/j.hydromet.2011.03.003
Li X, Li Z, Orefice M, Binnemans K (2019) Metal recovery from spent samarium–cobalt magnets using a trichloride ionic liquid. ACS Sustain Chem Eng 7:2578–2584. https://doi.org/10.1021/acssuschemeng.8b05604
Ola P-D, Matsumoto M, Kondo K (2017) Recovery of Fe and Mn from aqueous solution with solvent extraction and liquid membrane permeation using ionic liquids. Desalin Water Treat 75:325–330. https://doi.org/10.5004/dwt.2017.20406
Song Y, Tsuchida Y, Matsumiya M, Uchino Y, Yanagi I (2018) Separation of tungsten and cobalt from WC-Co hard metal wastes using ion-exchange and solvent extraction with ionic liquid. Miner Eng 128:224–229. https://doi.org/10.1016/j.mineng.2018.08.047
Lu J, Dreisinger D (2014) Two-stage countercurrent solvent extraction of copper from cuprous chloride solution: Cu(II) loading coupled with Cu(I) oxidation by oxygen and iron scrubbing. Hydrometallurgy 150:41–46. https://doi.org/10.1016/j.hydromet.2014.09.003
Choi I, Moon G, Lee J, Jyothi R-K (2018) Hydrometallurgical processing of spent selective catalytic reduction (SCR) catalyst for recovery of tungsten. Hydrometallurgy 178:137–145. https://doi.org/10.1016/j.hydromet.2018.04.011
Lai W, Liu M, Li C, Suo H, Yue M (2014) Recovery of a composite powder from NdFeB slurry by co-precipitation. Hydrometallurgy 150:27–33. https://doi.org/10.1016/j.hydromet.2014.08.014
Mikutta C, Frommer J, Voegelin A, Kaegi R, Kretzschmar R (2010) Effect of citrate on the local Fe coordination in ferrihydrite arsenate binding, and ternary arsenate complex formation. Geochim Cosmochim Acta 74:5574–5592. https://doi.org/10.1016/j.gca.2010.06.024
Esalah J, Weber M-W, Vera J-H (1999) Removal of lead from aqueous solutions by precipitation with sodium di-(n-octyl) phosphinate. Sep Purif Technol 18:25–36. https://doi.org/10.1016/S1383-5866(99)00046-5
Esalah J, Husein M-M (2008) Removal of heavy metals from aqueous solutions by precipitation-filtration using novel organo-phosphorus ligands. Sep Sci Technol 43:3461–3475. https://doi.org/10.1080/01496390802219661
Masambi S, Dorfling C, Bradshaw S (2016) Comparing iron phosphate and hematite precipitation processes for iron removal from chloride leach solutions. Miner Eng 98:14–21. https://doi.org/10.1016/j.mineng.2016.07.001
Izadi A, Mohebbi A, Amiri M, Izadi N (2017) Removal of iron ions from industrial copper raffinate and electrowinning electrolyte solutions by chemical precipitation and ion exchange. Miner Eng 113:23–35. https://doi.org/10.1016/j.mineng.2017.07.018
Luo W, Kelly S-D, Kemner K-M, Watson D, Zhou J, Jardine P-M, Gu B (2009) Sequestering uranium and technetium through co-precipitation with aluminum in a contaminated acidic environment. Environ Sci Technol 43:7516–7522. https://doi.org/10.1021/es900731a
Huo L, Li W, Lu L, Cui H, Xi S, Wang J, Zhao B, Shen Y, Lu Z (2000) Preparation structure and properties of three-dimensional ordered α-Fe2O3 nanoparticulate film. Chem Mater 12:790–794. https://doi.org/10.1021/cm990690+
Brown ASC, Hargreaves JSJ, Rijniersce B (1998) A study of the structural and catalytic effects of sulfation on iron oxide catalysts prepared from goethite and ferrihydrite precursors for methane oxidation. Catal Lett 53:7–13. https://doi.org/10.1023/A:1019016830208
Quddus M-S, Rahman M-L, Khanam J, Biswas B, Sharmin N, Ahmed S, Tahuran NAJM (2018) Synthesis and characterization of pigment grade red iron oxide from mill scale. Int Res J Pure Appl Chem 16:1–9. https://doi.org/10.9734/IRJPAC/2018/42935
Muller M, Villaba J-C, Mariani F-Q, Dalpasquale M, Lemos M-Z, Huila MFG, Anaissi F-J (2015) Synthesis and characterization of iron oxide pigments through the method of the forced hydrolysis of inorganic salts. Dyes Pigm 120:271–278. https://doi.org/10.1016/j.dyepig.2015.04.026
Bouargane B, Marrouche A, Issiouy S-E, Biyoune M-G, Mabrouk A, Atbir A, Bachar A, Bellajrou R, Boukbir L, Bakiz B (2019) Recovery of Ca(OH)2, CaCO3, and Na2SO4 from Moroccan phosphogypsum waste. J Mater Cycles Waste Manae 21:1563–1571. https://doi.org/10.1007/s10163-019-00910-9
Jung C-H, Osako M (2009) Water extraction with CO2 bubbling as pretreatment of melting-furnace fly ash for metal recovery. J Mater Cycles Waste Manag 11:65–72. https://doi.org/10.1007/s10163-008-0220-6
Acknowledgements
This work was supported by the Technology Innovation Program, funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) under contract number 10052751. This work was also supported by the “Human Resources Program in Energy Technology” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resources from the MOTIE (Korea) through the grant number 20174010201150.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, H.J., Yoon, S.W., Kim, Y.J. et al. Recovery of iron oxide and calcium chloride from an iron-rich chloride waste using calcium carbonate. J Mater Cycles Waste Manag 23, 222–230 (2021). https://doi.org/10.1007/s10163-020-01119-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01119-x