Integrative Taxonomy Reveals Two New Species of Stalked Barnacle (Cirripedia, Thoracica) From Seamounts of the Western Pacific With a Review of Barnacles Distributed in Seamounts Worldwide
- 1Department of Marine Organism Taxonomy & Phylogeny, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China
- 3Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology, Qingdao, China
- 4Key Laboratory of Marine Ecosystem and Biogeochemistry, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China
- 5University of Chinese Academy of Sciences, Beijing, China
- 6School of Oceanography, Shanghai Jiao Tong University, Shanghai, China
Dozens of samples of stalked barnacles were collected from deep-sea seamounts of the tropical western Pacific by remotely operated vehicles during two expeditions in 2017 and 2018. Integrative taxonomy indicates that they represent two new species belonging to the families Scalpellidae and Poecilasmatidae, respectively. In terms of morphology, Arcoscalpellum angularum sp. nov. is distinguished from congeneric species by the angular processes on the dorsum of its soft body and the absence of a caudal appendage, whereas Glyptelasma robustum sp. nov. differs from its congeners in its robust peduncle, semicircular concaved carina, and long filamentary appendages. The validity of the two new species is supported by genetic analyses inferred from COI gene sequences and geographic distribution. To date, very few seamounts in the oceans have been investigated for scientific purposes, and records about barnacles inhabiting seamounts are chaotic. A literature search reveals about 125 barnacle species recorded in seamounts mainly in the eastern and western regions of the Pacific Ocean. Existing data are insufficient for research on species speciation and diffusion; still more credible data on the distribution of barnacles in seamounts should be collected.
Introduction
Seamounts are widespread on the seafloors of oceans, in particular around oceanic ridges and submarine trenches. Their distinctive environmental conditions, such as their spatial features, hydrological characteristics, sedimentary characteristics, and so on, make seamounts unique habitats for the deep-sea marine benthos. However, because they are influenced by the surrounding environment, seamount habitats are highly heterogeneous and breed various ecosystems (Kvile et al., 2014). On account of their distinct ecosystems, seamounts attract great curiosity and interest from scholars of oceanology, ecology, and biology exploring the biodiversity, origin, diffusion, and connectivity of seamount biocenoses (Wilson and Kaufmann, 1987; Rogers, 1994; Stocks, 2004; Shank, 2010; Miller and Gunasekera, 2017). Seamounts are thought to be life oases or stepping-stones in the spread of marine organisms (Newman, 1986; Worm et al., 2003). To date, more than 2700 valid species have been identified inhabiting seamounts in the oceans, according to the Ocean Biodiversity Information System (OBIS1). Nevertheless, most records have focused on Cnidaria, Porifera, and Echinodermata; compared with these groups, the numbers of barnacle species distributed in seamounts are very low.
In recent years, the systematic and evolutionary biology of Cirripedia have developed substantially. Evidence from mineralogy, paleontology, shell morphology, and molecular analysis presents great challenges to the current system of classifying higher taxa in Thoracica (Lin et al., 2015; Gale, 2016a, 2019; Gale et al., 2019). In the meantime, many species collected from the deep sea have been found and identified (Chan et al., 2014; Shalaeva and Newman, 2016), and the deep-sea adaptation of barnacles has been explored (Gan et al., 2020). As one of the most species-rich taxa in the family Scalpellidae, the genus Arcoscalpellum contains 43 extant species inhabiting depths from 40 to 5250 m; they are mainly distributed in the deep water around the oceans, excluding the Arctic (Shalaeva and Boxshall, 2014). The genus Glyptelasma is a typical deep-sea inhabitant group of the family Poecilasmatidae with 11 valid species mainly attached to urchin, sponge, or hard substrata and distributed in the deep-sea seafloor of subtropical and tropical seas (Jones and Hosie, 2016; Gan and Li, 2019b).
In September 2017 and April 2018, two comprehensive survey cruises were executed at the Weijia Guyot and Huangyanxi seamount of the western Pacific by the Guangzhou Marine Geological Survey and Tongji University using the Haima and Ropos remotely operated vehicles (ROVs), respectively. During these cruises, dozens of specimens of stalked barnacle were collected. The specimens captured in Weijia Guyot show characters of the genus Arcoscalpellum, and the specimens captured in the Huangyanxi seamount present features of the genus Glyptelasma. Examination and integrative research indicate that they are new species to science.
Materials and Methods
Sample Collection and Morphological Examination
Arcoscalpellum specimens were collected from the roof deck of Weijia Guyot by the ROV Haima, and specimens of Glyptelasma were captured from the brae of the Huangyanxi seamount by the ROV Ropos (Figure 1). All specimens were preserved in 75% ethanol when taken on board and then deposited in the Sample Repository of the Second Institute of Oceanography (SRSIO), Ministry of Natural Resources of the People’s Republic of China, Hangzhou.
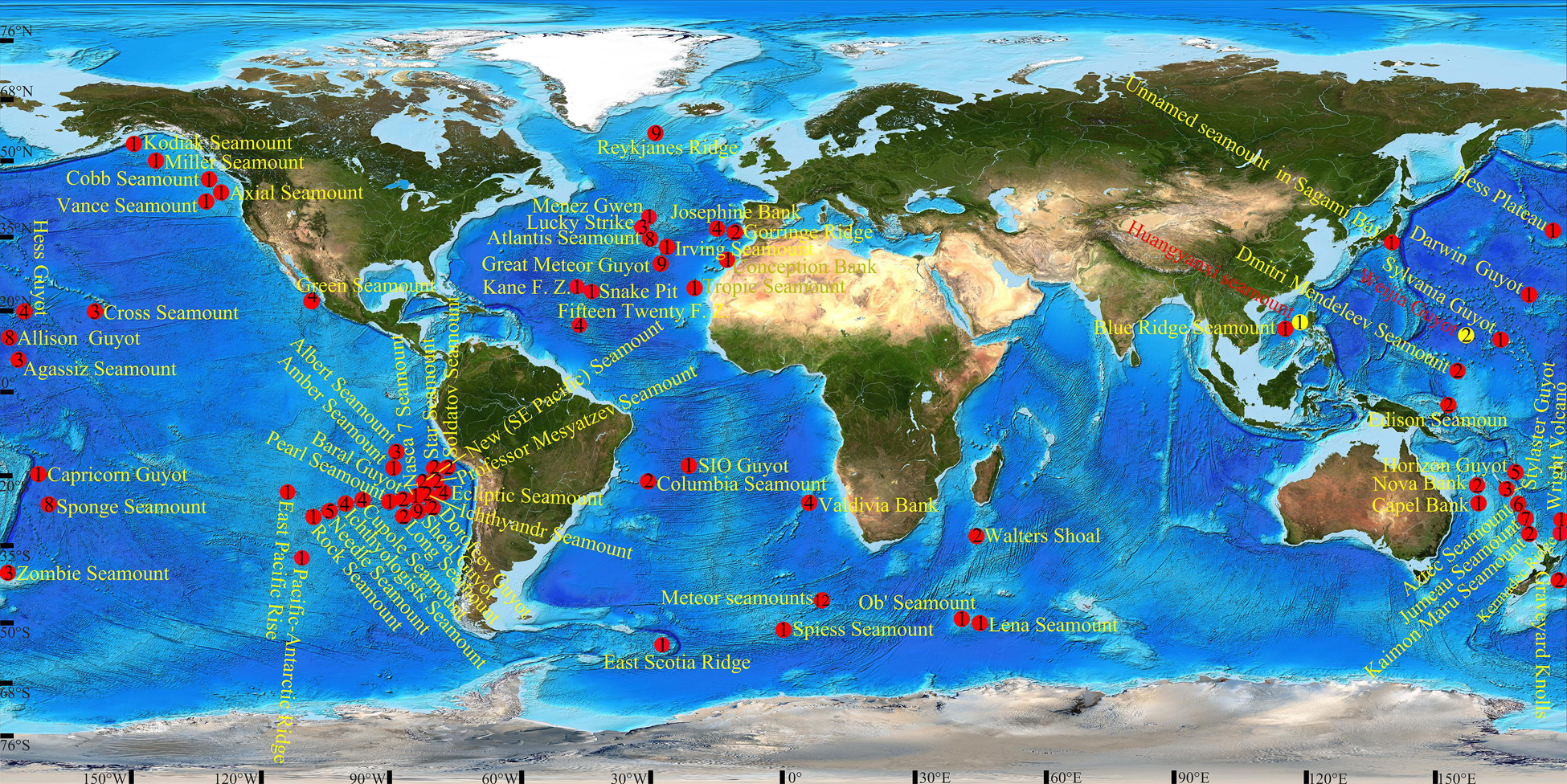
Figure 1. Locations of barnacles distributed in seamounts. Yellow circles indicate sites from which the new species were collected; Arabic numerals in circles indicate the number of barnacle species in the seamounts.
Specimens were dissected with a stereomicroscope (SMZ1500, Nikon, Japan) and photographed with a microscope (AZ100, Nikon). Specimen measurements are as follows: capitular length (CL), capitular width (CW), peduncular length (PL).
DNA Extraction, Sequencing, and Phylogenetic Analyses
Total genomic DNA was extracted from prosoma of specimens with a Marine Animals gDNA Purification Kit (Biomiga, San Diego, CA, United States) following the manufacturer’s instructions. Mitochondrial COI and 16S rRNA gene fragments were amplified from the diluted DNA with primers L1490/H2198 and 16S-AR/1472, respectively (Folmer et al., 1994; Crandall and Fitzpatrick, 1996). Polymerase chain reactions were performed in a 50 μL volume containing 25 μL Premix Taq (TaKaRa, Kusatsu, Japan), 1 μL each of the forward and reverse primers (10 μM), 3 μL DNA template, and 20 μL ultrapure water. The reaction profile consisted of initial denaturation at 94°C for 5 min, 35 cycles of denaturation at 94°C for 30 s, annealing at 50–52°C for 40 s, elongation at 72°C for 50 s, and a final extension at 72°C for 10 min. The reaction products were purified with a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and bidirectionally sequenced with the same primers with an ABI 3730xl Analyzer (Applied Biosystems, Foster City, CA, United States). Sequences were checked and proofread by ContigExpess 6.0 (a component of Vector NTI Suite 6.0, LifeTechnologies, Carlsbad, CA, United States).
As the 16S rRNA sequence is scarce in relatives of the target species, genetic analyses are mainly based on the COI sequence, which includes 38 sequences with Ibla cumingi as the out-group. The sequence data were aligned with MUSCLE 3.8 (Edgar, 2004) and amended manually. The best fitting nucleotide base substitution model (GTR + I + G) for the alignment data were determined with Modeltest 3.7 (Posada and Crandall, 1998). A maximum likelihood tree was constructed using PhyML 3.1 (Guindon et al., 2010) with 1000 bootstrap reiterations. A Bayesian inference tree was constructed with MrBayes 3.2.7 (Ronquist et al., 2012); the Markov chains were run for 3,000,000 generations, sampled every 3000 generations. The first 25% of trees were discarded as burn-in, and the remaining trees were used to construct the 50% majority rule consensus tree with posterior probabilities. Kimura’s 2-parameter genetic distances among scalpellid species were calculated with MEGA X (Kumar et al., 2018).
Results
Taxonomy of Arcoscalpellum angularum sp. nov.
Infraclass Cirripedia Burmeister, 1834
Superorder Thoracica Darwin, 1854
Order Scalpelliformes Buckeridge & Newman, 2006
Family Scalpellidae Pilsbry, 1907
Genus Arcoscalpellum Hoek, 1907
Arcoscalpellum angularum sp. nov.
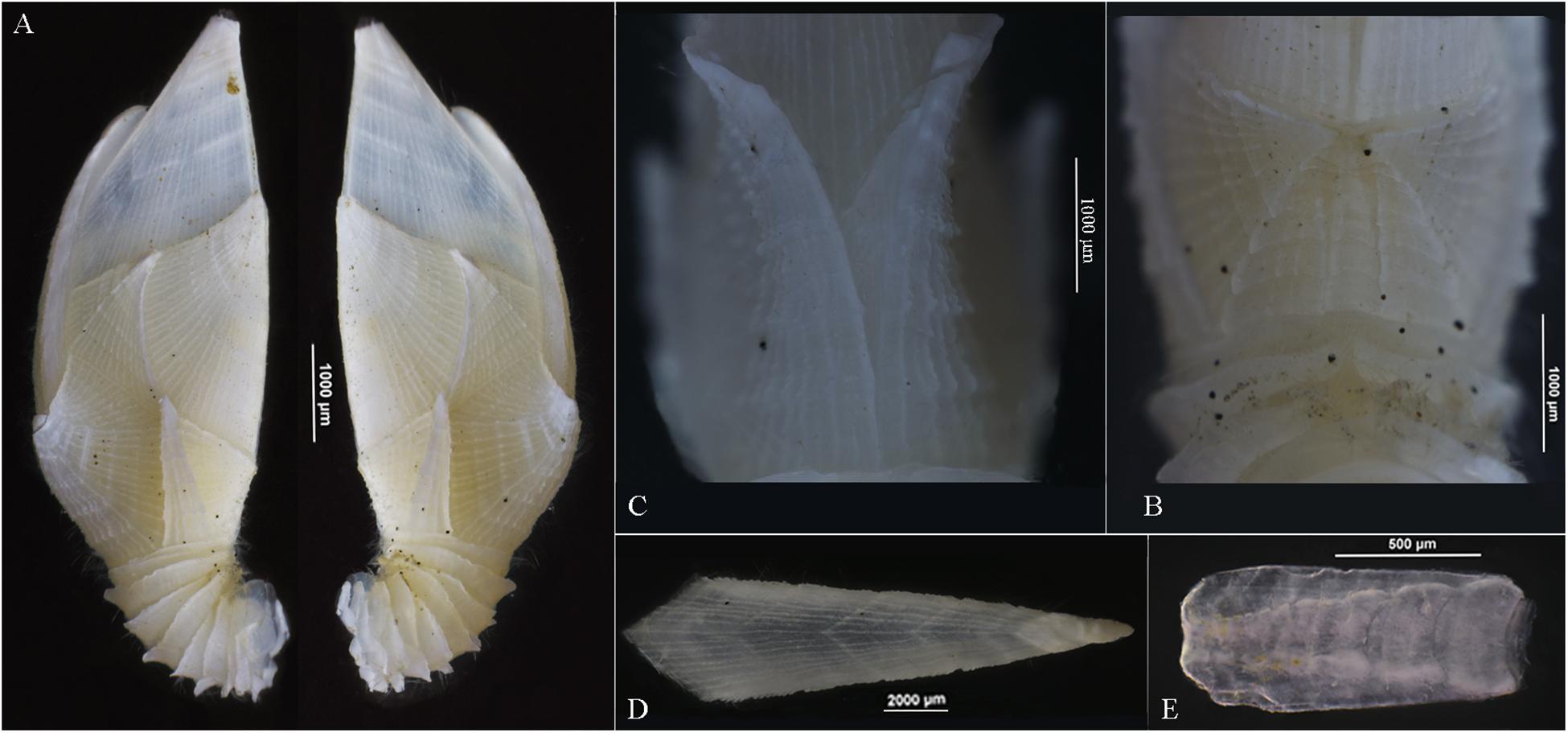
Figure 2. Arcoscalpellum angularum sp. nov. holotype. (A) Lateral views. (B) Rostrum in situ. (C) Carinal view of carinolatus. (D) Carina. (E) Rostrum.

Figure 3. Arcoscalpellum angularum sp. nov. holotype. (A) Inner edge of labrum. (B) Palp. (C) Mandible. (D) Inferior angle of mandible. (E) Maxillule. (F) Maxilla.
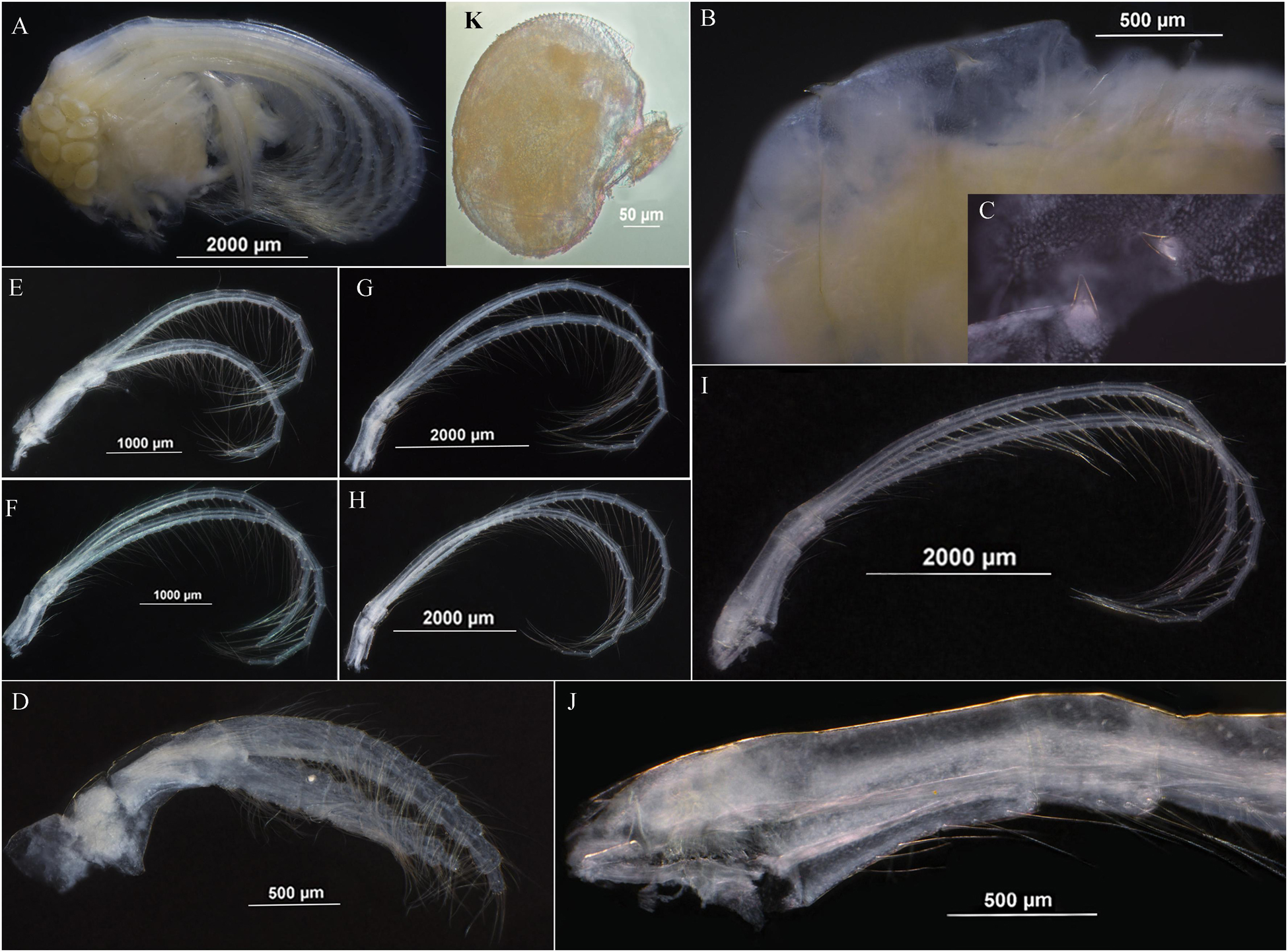
Figure 4. Arcoscalpellum angularum sp. nov. holotype. (A) Soft body. (B,C) Angular processes on dorsum of thorax. (D) Cirrus I. (E) Cirrus II. (F) Cirrus III. (G) Cirrus IV. (H) Cirrus V. (I) Cirrus VI. (J) Basal part of cirrus VI. (K) Dwarf male.
ZooBank Registration LSID
urn:lsid:zoobank.org:act:3530D998-F4B1-4DD6-990D-2DCCE9565AD3.
Material Examined
Holotype: SRSIO17090311, Stn. MCROV06, 156°41′15.72″E, 12°47′22.2″N, depth 1935 m, roof deck of Weijia Guyot, attaching to shell fragment, coll. crew of ROV Haima, Dayang 41B cruise, September 21, 2017, measurements CL 8.6 mm, CW 4.5 mm, PL 3.2 mm. Paratype: SRSIO17090312, five specimens, same information as the holotype collected, attaching to shell fragment or clastic rocks, measurements CL 7.2–10.2 mm, CW 3.6–4.9 mm, PL 2.5–3.8 mm.
Diagnosis
Small-sized scalpellid barnacle. Capitulum elongated, 14 fully calcified capitular plates, all plates with strong longitudinal ridges on surface. Inframedian latus triangular, very narrow, umbo apical. Carinolatus pentagonal, higher than wide, umbo sub-basal of carina. Dorsum of thoracic body with two pairs of angular process. Caudal appendage absent.
Description
Capitulum elongated, 2.3–2.5 times as long as wide, capitular plates 14, fully calcified, white, all with strong longitudinal ridges on surface (Figure 2A). Tergum quadrilateral, carinal margin curved at distal third, occludent margin straight, umbo apex, thickened (Figure 2A). Scutum fan-shape, tergal margin slightly concave, occludent margin slightly convex, lateral and basal margins consecutive and curved equably, umbo apical (Figure 2A). Upper latus almost isosceles trapezium, scutal margin concaved and longest, other margins straight, umbo apical, thickened, medial apico-basal ridge prominent (Figure 2A). Rostrolatus quadrilateral, height two times width, basal margin shortest, apico-basal ridge distinct (Figures 2A,B). Inframedian latus triangular, narrow, reaching the upper latus, more than three times as high as wide, umbo apical, thickened and protruded laterally (Figure 2A). Carinolatus pentagonal, higher than wide, umbo sub-basal of carina, thickened, slightly exceeding beyond carina, two carinolatus intersected once at the middle (Figures 2A,C). Carina equally arched, tectum with V-shape and longitudinal ridges, basal triangular (Figures 2A,D). Rostrum rectangle, covered by paired rostrolatus obliquely (Figures 2B,E). Peduncle short, covered by laterally elongated and produced scales (Figure 2A).
Inner edge of labrum with single row of teeth (Figure 3A). Palp small with long setae along apical margin (Figure 3B). Mandible with three large acute teeth, the one near base largest and far away from others, inferior angle blunt, armed with closely long spicules (Figures 3C,D). Maxillule cutting edge stair-like, furnished with long cuspidate setae (Figure 3E). Anterior margin of maxilla without notch, upper and anterior margins with long setae (Figure 3F).
Soft body without penis (Figure 4A) with two pairs of angular processes on dorsum of thorax, acutely pointed (Figures 3B,C). Cirrus I shorter than cirri II–VI, anterior ramus slightly shorter than posterior, 9 segments, posterior ramus 10 segments, both rami furnishing with long setae (Figures 4A,D). Cirri II–VI with rami nearly equal, each rami segment armed with three pairs of long setae and one pair of proximal short setae on inner edge with one pair of setae on outer margin of joint (Figures 4E–I). Basal surface of cirrus VI protopodite smooth, caudal appendages absent (Figure 4J).
Dwarf male oval, without plates, located at inner occludent margin of scutum, attaching to mantle (Figure 4K). Egg olive shape, egg mass located at dorsum of soft body (Figure 4A).
Habitat and Distribution
Roof deck of deep-sea mount, attaching to shell fragments or rocks. Currently only known from the type locality, Weijia Guyot.
GenBank Accession Numbers of Holotype
MT675948 (COI), MT668953 (16S rRNA).
Etymology
The specific name angularum derived from the word “angular,” referring to the angular process located at the dorsum of the soft body.
Remarks
Like most of the Arcoscalpellum species, A. angularum sp. nov. is dioecious; its dwarf male is extremely vestigial and small, attaching to the mantle of the female. In general, the filamentary appendage is absent from the Arcoscalpellum species even from the scalpellid species (Zevina, 1981b). However, the current species has two pairs of angular processes on the dorsum of the thoracic body; this kind of process is only reported in Litoscalpellum spinosus (Chan et al., 2010). In contrast, nearly all Arcoscalpellum species have a single- or multi-segment caudal appendage (Zevina, 1978). Even if it is vestigial, some trace should be observed, such as Arcoscalpellum ciliatum and Arcoscalpellum gryllum, but no vestigial trace was observed in the present species under the microscope.
Taxonomy of Glyptelasma robustum sp. nov.
Infraclass Cirripedia Burmeister, 1834
Superorder Thoracica Darwin, 1854
Order Lepadiformes Buckeridge & Newman, 2006
Family Poecilasmatidae Annandale, 1909
Genus Glyptelasma Pilsbry, 1907
Glyptelasma robustum sp. nov.
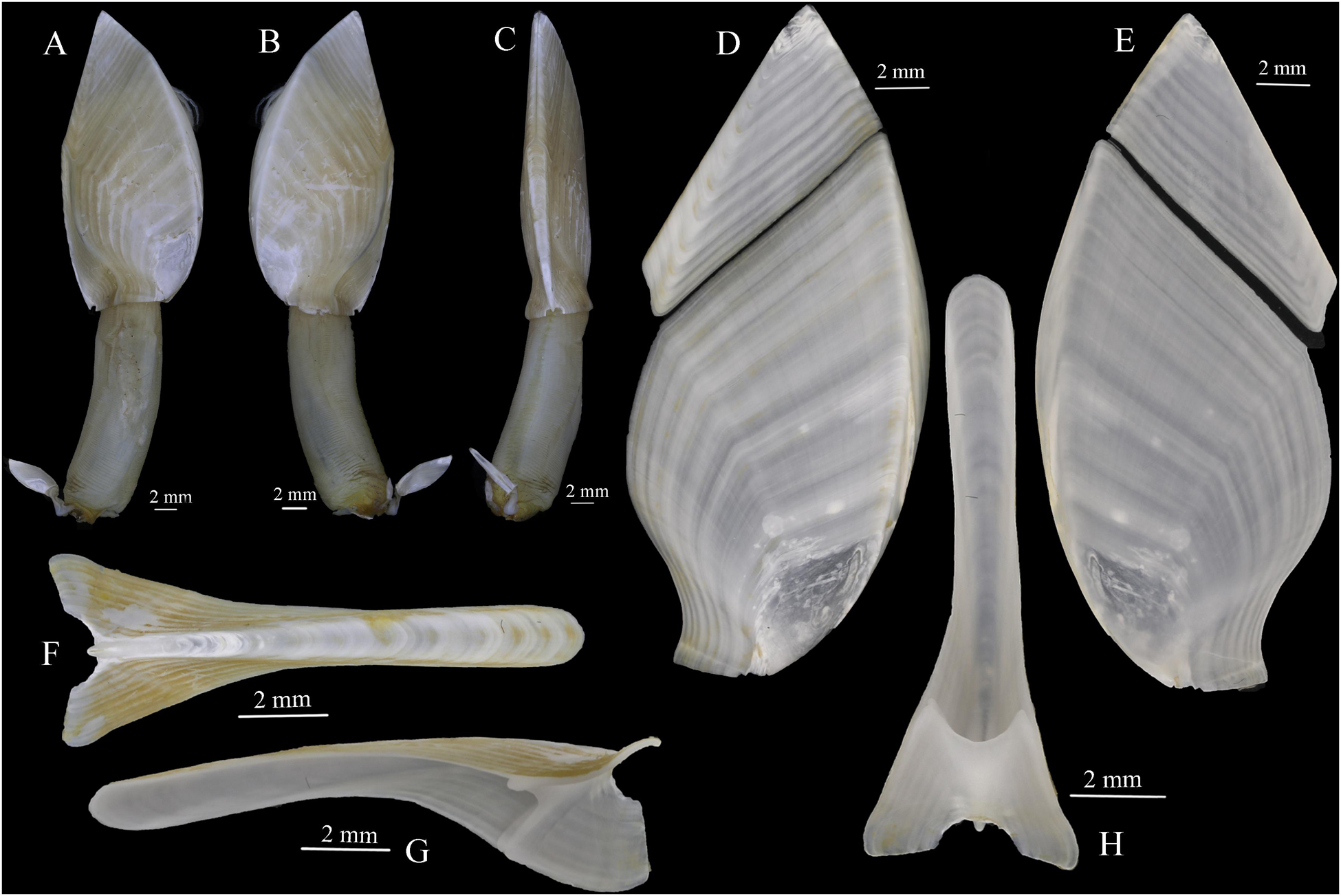
Figure 5. Glyptelasma robustum sp. nov. holotype. (A,B) Lateral views. (C) Carinal view. (D) Exterior view of tergum and scutum. (E) Inner view of tergum and scutum. (F) Exterior view of carina. (G) Lateral view of carina. (H) Inner view of carina.
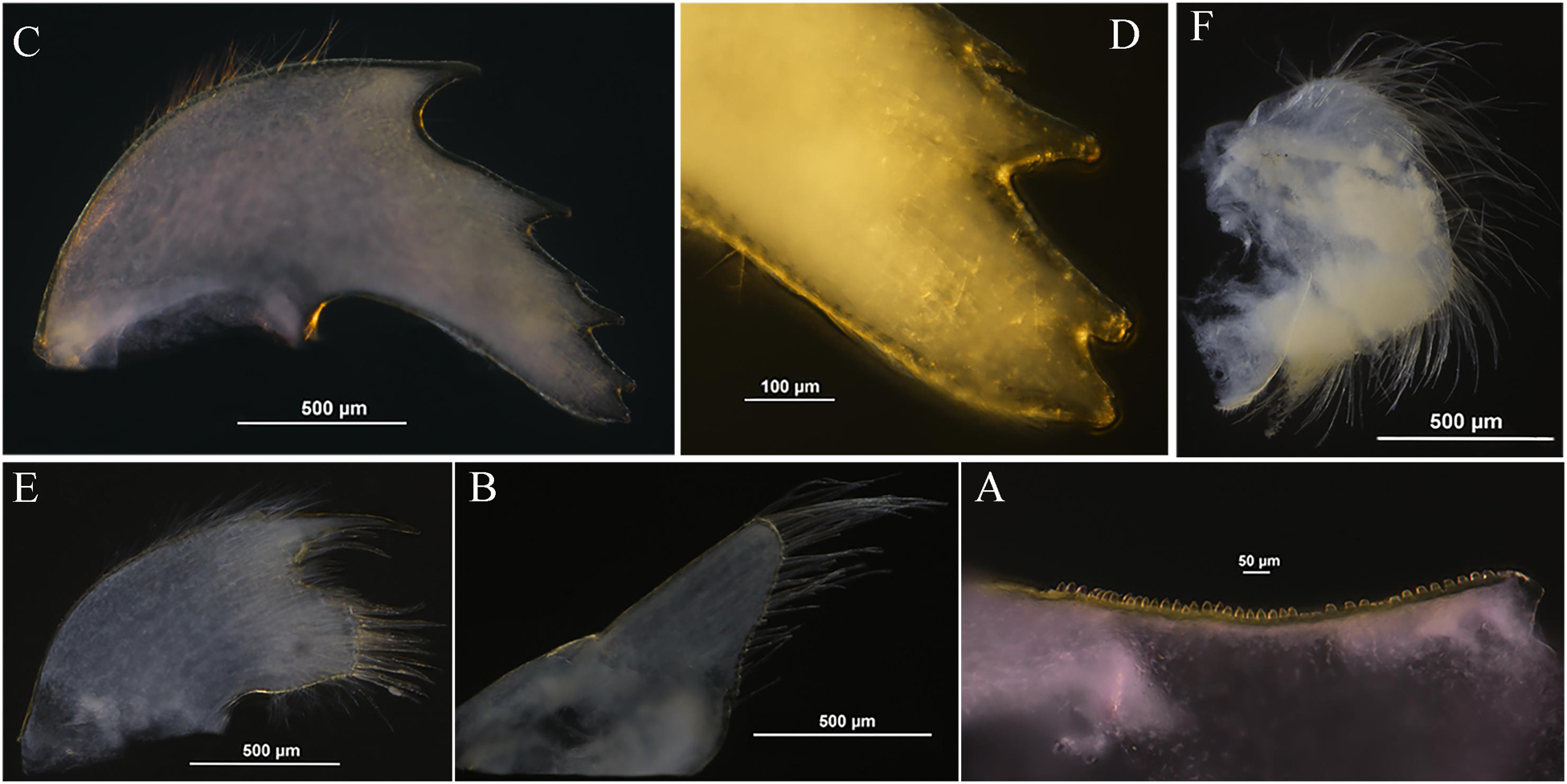
Figure 6. Glyptelasma robustum sp. nov. holotype. (A) Inner edge of labrum. (B) Palp. (C) Mandible. (D) Inferior angle of mandible. (E) Maxillule. (F) Maxilla.
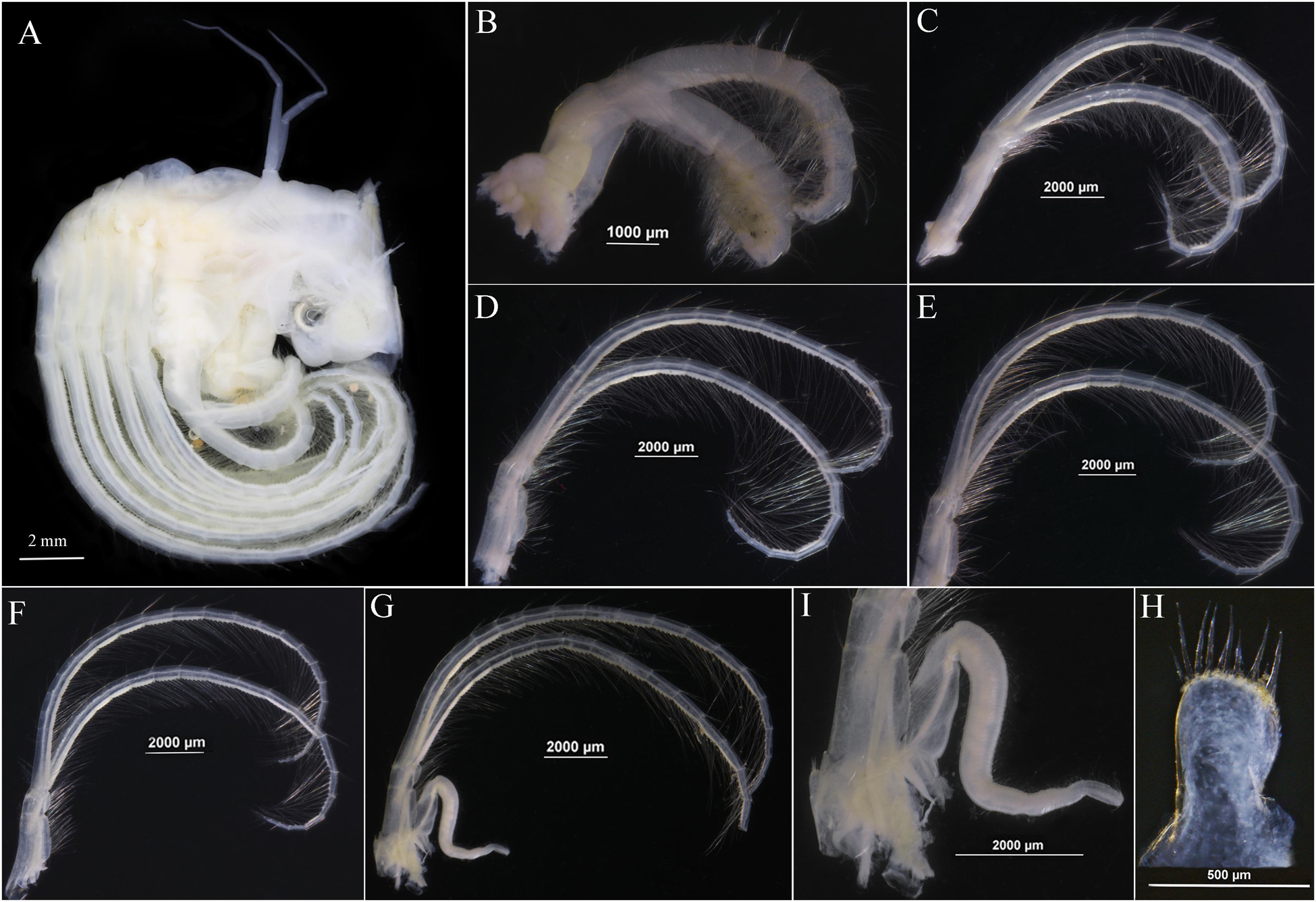
Figure 7. Glyptelasma robustum sp. nov. holotype. (A) Soft body. (B) Cirrus I. (C) Cirrus II. (D) Cirrus III. (E) Cirrus IV. (F) Cirrus V. (G) Cirrus VI. (H) Caudal appendage. (I) Penis.
ZooBank Registration LSID
urn:lsid:zoobank.org:act:C9242D9A-7356-4D64-8D60-2F73064017E1.
Material Examined
Holotype: SRSIO18040310, Stn. R2055, 117°34.8684′E, 15°16.9688′N, depth 2987 m, brae of Huangyanxi seamount, attaching to coral limb, coll. crew of ROV Ropos, April 27, 2018, measurements CL 29.8 mm, CW 13.6 mm, PL 26.0 mm. Paratype: SRSIO18040311, 12 specimens, same information as the holotype collected, measurements CL 4.7–32.6 mm, CW 2.5–14.3 mm, PL 1.5–26.8 mm.
Diagnosis
Large-sized poecilasmatid barnacle. Capitulum with five fully calcified plates. Basal margins of scutum and carina consecutive, straight. Lower half of carina expanded laterally; basal margin semicircular concaved. Peduncle robust, more than half the length of capitulum. Dorsum of soft body with one pair of long tapering filamentary appendages.
Description
Capitulum leaf-like, slightly swollen bilaterally, bottom margin straight, surface smooth, composed of five closely connected plates, white, growth lines visible, none serrated (Figures 5A–C). Tergum quadrilateral, all margins straight other than carinal margin slightly concave, apex acute, apico-bottom ridge bland (Figures 5D,E). Scutum wing-shaped, tergal margin straight, occludent margin arcuate, carinal margin sigmoid, basal margin straight, longer than and forming a straight line with the basal margin of carina, apico-umbo ridge distinct, ridge from umbo to tergal-carinal angle faint (Figures 5D,E). Carina slightly arched, two sides of tectum curved inward, lower half of carina evenly expanded bilaterally, basal margin semicircular concaved, inner septum with upper margin semicircular concaved (Figures 5F–H). Peduncle long and robust, about 0.62–0.87 times as long as capitulum, width only slightly shorter than the bottom margin of capitulum, without scales, with annular wrinkles (Figures 5A,B).
Labrum with single row of sub-quadrate teeth on inner edge (Figure 6A). Palp broad, with long setae along apical and interior margin (Figure 6B). Mandible with four acute teeth excluding inferior angle, first tooth largest and third one smallest, inferior angle with one blunt tooth upper, apex rough (Figures 6C,D). Maxillule cutting edge with notch, one long spine and two cuspidate setae above notch, a dozen long cuspidate setae below notch (Figure 6E). Anterior margin of maxilla without notch, surface furnished with long setae (Figure 6F).
Soft body with one pair of long tapering filamentary appendages on dorsum (Figure 7A). Cirrus I shorter than and away from cirri II–VI, both rami sub-equal, anterior ramus 11 segments, posterior ramus 13 segments, surface furnished with long setae, basal portion of cirrus I without filamentary process (Figure 7B). Cirri II–VI with rami nearly equal, each rami segment armed with five or six pairs of long setae on inner edge (Figures 7C–G). Caudal appendages short, thumb-like, one segment, with nine terminal setae (Figures 7H,I). Penis long, tapering, surface with annular wrinkles and pervasive setae (Figure 7I).
Habitat and Distribution
Brae of Huangyanxi seamount, attaching to coral limb. Currently only known from the type locality, Huangyanxi seamount.
GenBank Accession Numbers of Holotype
MT675949 (COI), MT668952 (16S rRNA).
Etymology
The specific name robustum derived from the word “robust,” referring to the thick and strong form of the new species, especially the robust peduncle.
Remarks
There are 11 valid Glyptelasma species listed in the World Register of Marine Species (WoRMS2) at present. Glyptelasma annandalei, Glyptelasma hamatum, Glyptelasma orientale, Glyptelasma pilsbryi, and Glyptelasma rectum were once reported from seamount habitat (Rao and Newman, 1972; Young, 1998a; Mironov and Krylova, 2006). The present new species is the first Glyptelasma species described from a deep-sea seamount habitat. However, if it is endemic to the seamount, more investigation and extensive collection from the deep sea is needed in the future. Moreover, most Glyptelasma species mainly attach to urchins; some can attach to sponges, cables, and hard substrata (Pilsbry, 1907; Jones and Hosie, 2016). The present new species and Glyptelasma carinatum attach to corals.
Genetic Results
The COI and 16S rRNA sequences of the holotypes of the two new species have been deposited in GenBank3. The phylogenetic tree recovers G. robustum sp. nov. with well-supported values in the Poecilasmatidae clade and A. angularum sp. nov. with less supported values in the Scalpellidae clade (Figure 8). Obviously, the monophyly of genus Arcoscalpellum is not supported by the phylogenetic tree; a similar result was obtained by Linse et al. (2013) and Lin et al. (2015). A complete matrix of Kimura’s 2-parameter genetic distances among scalpellid species can be found in Supplementary Table 1.

Figure 8. Bayesian inference tree recovered from COI sequence data. Numbers at nodes represent posterior probabilities/bootstrap values, and numbers less than 0.50 or 50 are shown as –.
Discussion
Systematic State of Arcoscalpellum angularum sp. nov.
The Scalpellidae is the most diverse group among the order Lepadiformes, but its current taxonomy is in a state of flux, especially for the genus Scalpellum and its closely related genera, such as Arcoscalpellum, Trianguloscalpellum, Litoscalpellum, and so on, the monophyly of these genera were rejected by recent phylogenetic analyses (Linse et al., 2013; Lin et al., 2015; Gale, 2016b). One of the reasons for this problem is that the taxonomy is based on a few morphological characters of plates. Integrative taxonomy is a promising method of overcoming the defects of traditional morphological taxonomy. It can use any information about a taxon, like morphology, genetics, ecological niche, auxology, physiology, distribution, habitat, and so on. Nevertheless, encompassing everything may result in less efficient, even unsustainable taxonomic research. Even so, using an approach that integrates morphology, molecular sequence, habitat, and distribution is a realizable and reliable method of describing new taxa, in particular for cryptic species (Gan and Li, 2019a).
Although the status of genus Arcoscalpellum is under question, it is retained to encompass the scalpellid species that have an apical umbo, a triangular inframedian latus reaching to the upper latus and a carinolatus with the umbo in the middle of the carinal edge. Thus, the present new species is assigned to Arcoscalpellum based on diagnostic characters of the genus, and the validity of the species is supported by morphological and molecular data. In terms of morphology, A. angularum sp. nov. is closely related to Arcoscalpellum compositum, Arcoscalpellum floccidum, Arcoscalpellum galapaganum, Arcoscalpellum gryllum, and Arcoscalpellum radiatum. They all have well-ridged plates, a lengthened triangular inframedian latus, a high carinolatus, and a vestigial caudal appendage. A. angularum sp. nov. differs from A. compositum in its inframedian latus (well ridged vs. smooth in A. compositum), its rectangle rostrum (large vs. small), and its peduncle (encompassing elongated and produced scales vs. scattering small oval scales); furthermore, A. compositum is distributed in the Puerto Rico Trench in the North Atlantic at a depth of more than 5000 m (Zevina, 1975, 1981a; Poltarukha, 2011). The new species differs from A. floccidum and A. galapaganum in the ornamentation of the inframedian latus and rostrolatus (with vs. without longitudinal ridges) and the linkage of the bilateral carinolatus (single-intersected vs. multi-intersected). It also differs from A. floccidum in the inferior angle of the mandible (with bunched blunt spicules vs. with dispersive acute spines) (Pilsbry, 1907; Zevina, 1975, 1981a). A. floccidum is also distributed in the Puerto Rico Trench in the North Atlantic at a depth of more than 5000 m (Zevina, 1975, 1981a), and A. galapaganum has only been reported in the Galapagos Islands in the southeastern Pacific at a depth of 1141 m (Pilsbry, 1907; Zevina, 1981b; Jones, 2007). A. angularum sp. nov. can be distinguished from A. gryllum by the linkage of the bilateral carinolatus (single-intersected vs. multi-intersected), the inferior angle of the mandible (with bunched blunt spicules vs. bifid, with dispersive small teeth), and the peduncle (encompassing elongated and produced scales vs. scattering small oval scales); moreover, A. gryllum is endemic to Australia and is found at a depth of 4850 m (Zevina, 1981b; Jones, 2012). The new species can be distinguished from A. radiatum by the ornamentation of the inframedian latus (with vs. without longitudinal ridges), the linkage of the bilateral carinolatus (single-intersected vs. multiply interdigitated), and the inferior angle of the mandible (with bunched blunt spicules vs. strongly denticulate); A. radiatum was reported in the Mid-Pacific Mountains (18°30′N, 179°36′W) and the Clarion-Clipperton region (13°55.612′N, 129°05.519′W) at a depth of 1413–4680 m (Rao and Newman, 1972; Poltarukha and Mel’nik, 2012). In addition, A. angularum sp. nov. is distinguishable from all congeneric species by the angular processes on the dorsum of its soft body and the absence of a caudal appendage.
The molecular analyses support A. angularum sp. nov. being a distinct species both in the phylogenetic tree (Figure 8) and in terms of genetic distance (Supplementary Table 1). The genetic divergence of the COI sequence between A. angularum sp. nov. and closely related species ranges from 26.73% (Arcoscalpellum chiliense) to 37.69% (Arcoscalpellum africanum). The average genetic divergence between A. angularum sp. nov. and closely related species is 31.10%, greater than the average interspecific genetic divergence (28.00%, calculated from all the scalpellid species in this study).
Systematic State of Glyptelasma robustum sp. nov.
Based on features of the carina and scutum, Glyptelasma species can be divided into two groups. G. robustum sp. nov. belongs to the group with basal margins of the carina and scutum organized in a straight line. In this group, the capitulum of G. robustum sp. nov. closely resembles that of G. carinatum, G. hamatum, G. orientale, and Glyptelasma subcarinatum. However, the new species differs from these species in its long and robust peduncle, which is unique among all Glyptelasma species as well. G. robustum sp. nov. is further distinguished from G. carinatum by its carina (basal margin semicircular concaved vs. medially convex) and filamentary appendage (two long filamentary appendages vs. dozens of filamentary appendages) (Hoek, 1883; Zevina, 1982; Young, 1999). The distinctions between G. robustum sp. nov. and G. hamatum are also in the carina (basal margin semicircular concaved vs. convex) and filamentary appendage (two long filamentary appendages vs. two short hook-like processes) (Calman, 1919). The filamentary appendages of G. orientale are most similar to those of G. robustum sp. nov., but G. orientale also has a pair of short finger-like appendages at the base of the first cirrus that are absent from G. robustum sp. nov.; furthermore, the basal margin of the carina in G. orientale and G. subcarinatum is straight, unlike that of G. robustum sp. nov. (Pilsbry, 1907; Calman, 1919).
In terms of distribution and habitat, G. carinatum and G. subcarinatum were originally described in the western Atlantic Ocean; G. subcarinatum attached to the spines of a sea urchin at a depth of 2894 m, whereas G. carinatum was found at 713–768 m (Hoek, 1883; Pilsbry, 1907). The type locality of G. hamatum and G. orientale was the southwestern Pacific Ocean at a depth ranging from 700 to 2700 m, and they attached to deep-sea telegraph cables (Calman, 1919). In subsequent reports, G. carinatum and G. hamatum were also recorded in the Indian Ocean and Atlantic Ocean (Young, 1999; Jones and Hosie, 2016), and the former can attach to corals (Pilsbry, 1907). Moreover, although the DNA sequence resource of Glyptelasma species is rare at present, the validity of G. robustum sp. nov. is supported by the present phylogenetic tree (Figure 8).
Barnacles (Thoracica) Distributed in Seamounts
Except for a few groups, such as genera Lepas, Coronula, Chelonibia, and so on, which can passively migrate with their hosts, most barnacles cannot move or have slight movement along substrate in their adult stage (Moriarty et al., 2008). Meanwhile, deep-sea barnacles are indicated that have a slow growth rate (Yusa et al., 2018) and long larval development (Ozaki et al., 2008), which might result in greater population and larger geographical distribution. Thus, barnacles are excellent materials for researching speciation and diffusion in the ocean. Researching barnacles in seamounts is even more informative as seamounts play a crucial role in the speciation and diffusion of marine organisms (Newman, 1986; Worm et al., 2003). However, data on barnacles distributed in seamounts are incomplete and scattered. Of the 18,153 records in the OBIS database (i.e., the Seamounts Online version 2005-1 data set), we only found 196 referring to barnacles, and some names of taxa are invalid from the current taxonomic schedule. Compiling from the OBIS database and other publications (Zullo and Newman, 1964; Rao and Newman, 1972; Newman, 1979; Zevina, 1981a, 1983; Jones, 1993; Young, 1998a, b, 1999, 2001, 2002; Buckeridge, 2000, 2009; Southward and Jones, 2003; Stocks, 2004; Tunnicliffe and Southward, 2004; Yamaguchi et al., 2004; Southward, 2005; Mironov and Krylova, 2006; Poltarukha and Zevina, 2006; Newman and Jones, 2011; Buckeridge et al., 2013; Kolbasov et al., 2017; Lobo and Tuaty-Guerra, 2017), there are approximately 125 barnacle species distributed in seamounts up to now, including the present two new species (Supplementary Table 2), and most occurrence records focus on the seamounts in the eastern and western regions of the Pacific Ocean (Figure 1). The family Scalpellidae is the dominant group, with 31 species distributed in seamounts, and the Meteor seamounts have the greatest number of barnacle species: about 12.
Wilson and Kaufmann (1987) indicate that the seamount biota can be classified into four main biogeographic categories, i.e., provincial, widespread to cosmopolitan, exotic, and endemic. On the basis of the present data (Supplementary Table 2), Arcoscalpellum michelottianum and Metaverruca recta are undoubtedly cosmopolitan species occurring in the Pacific, Atlantic, and Indian Oceans. But it is premature to determine the categories of other species, especially endemic species, because no more than 5% of seamounts have been investigated at present (Kvile et al., 2014), and the investigated data referring to barnacles are merely preliminary, most of the data were only obtained from few sampling sites in a seamount. The lack of data presents challenges for research on the speciation and diffusion of barnacle species, as having more information enables researchers to make more credible conclusions. Therefore, the current priority is to accumulate sufficient knowledge of the distribution of barnacles in seamounts.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MT675949; https://www.ncbi.nlm.nih.gov/genbank/, MT668952; https://www.ncbi.nlm.nih.gov/genbank/, MT675948; https://www.ncbi.nlm.nih.gov/genbank/, MT668953.
Author Contributions
XL and CW conceived and coordinated the project. ZG performed the morphological work and molecular analyses. ZG and PX drafted the manuscript. XL and CW revised the manuscript. All authors approved the final manuscript.
Funding
This work was supported financially by the China Ocean Mineral Resources R&D Association Special Foundation (Nos. DY135-E2-2-03, DY135-E2-2-06, and DY135-E2-3-04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the crews of the Haima and Ropos ROVs for their help in collecting the specimens. Thanks are due to Dr. Oleg P. Poltarukha and Dr. Temir A. Britayev, who kindly furnished us with some Russian publications. Sincere thanks are extended to Dr. Diana S. Jones and Dr. Kate Shalaeva, who kindly offered us literature and conferred on the taxonomy of Scalpellidae.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.582225/full#supplementary-material
Supplementary Table 1 | Kimura’s 2-parameter genetic distances based on COI sequence among scalpellid species.
Supplementary Table 2 | List of Thoracica barnacle species occurring in the seamounts worldwide.
Footnotes
- ^ https://obis.org/dataset/fa9bc19f-f98a-4278-ab99-c6d8ef4966c7
- ^ http://www.marinespecies.org
- ^ https://www.ncbi.nlm.nih.gov
References
Buckeridge, J. S. (2000). A new deep-sea vent barnacle, Neolepas osheai sp. nov. (Cirripedia: Thoracica) from the Brothers Caldera, South-west Pacific Ocean. New Zeal. J. Mar. Fresh. 34, 409–418. doi: 10.1080/00288330.2000.9516944
Buckeridge, J. S. (2009). Ashinkailepas kermadecensis, a new species of deep-sea scalpelliform barnacle (Thoracica: Eolepadidae) from the Kermadec Islands, southwest Pacific. Zootaxa 2021, 57–65. doi: 10.11646/zootaxa.2021.1.4
Buckeridge, J. S., Linse, K., and Jackson, J. A. (2013). Vulcanolepas scotiaensis sp. nov., a new deep-sea scalpelliform barnacle (Eolepadidae: Neolepadinae) from hydrothermal vents in the Scotia Sea, Antarctica. Zootaxa 3745, 551–568. doi: 10.11646/zootaxa.3745.5.4
Calman, W. T. (1919). On barnacles of the genus Megalasma from deep-sea telegraph cables. Ann. Mag. Nat. Hist. Mus. 4, 361–374. doi: 10.1080/00222931908673905
Chan, B. K. K., Corbari, L., Moreno, P. A., and Jones, D. S. (2014). Two new deep-sea stalked barnacles, Arcoscalpellum epeeum sp. nov. and Gymnoscalpellum indopacificum sp. nov., from the Coral Sea, with descriptions of the penis in Gymnoscalpellum dwarf males. Zootaxa 3866, 261–276. doi: 10.11646/zootaxa.3866.2.5
Chan, B. K. K., Prabowo, R. E., and Lee, K. S. (2010). North West Pacific deep-sea barnacles (Cirripedia, Thoracica) collected by the TAIWAN expeditions, with descriptions of two new species. Zootaxa 2405, 1–47.
Crandall, K. A., and Fitzpatrick, J. F. Jr. (1996). Crayfish molecular systematics: using a combination of procedures to estimate phylogeny. Syst. Biol. 45, 1–26. doi: 10.1093/sysbio/45.1.1
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Gale, A. (2016a). Origin and phylogeny of the Cretaceous thoracican cirripede family stramentidae. J. Syst Palaeontol. 14, 653–702. doi: 10.1080/14772019.2015.1091149
Gale, A. (2016b). Phylogeny of the deep-sea cirripede family Scalpellidae (Crustacea, Thoracica) based on shell capitular plate morphology. Zool. J. Linn. Soc. 176, 266–304. doi: 10.1111/zoj.12321
Gale, A. (2019). Stalked barnacles (Cirripedia, Thoracica) from the Upper Jurassic (Tithonian) Kimmeridge Clay of Dorset, UK; palaeoecology and bearing on the evolution of living forms. Pac. Geol. Assoc. 130, 355–365. doi: 10.1016/j.pgeola.2018.01.005
Gale, A., Schweigert, G., Keupp, H., and Röper, M. (2019). Thoracican cirripedes (Crustacea) from the Kimmeridgian of Brunn and Nusplingen (southern Germany), and their bearing on the origin of calanticid and scalpellid barnacles. N. Jb. Geol. Paläont. Abh. 293, 1–17. doi: 10.1127/njgpa/2019/0822
Gan, Z., and Li, X. (2019a). Recognizing two new Hippolyte species (Decapoda, Caridea, Hippolytidae) from the South China Sea based on integrative taxonomy. PeerJ 7:e6605. doi: 10.7717/peerj.6605
Gan, Z., and Li, X. (2019b). Report on four deep-water barnacles (Cirripedia, Thoracica) from the north west Pacific, with remarks on Trianguloscalpellum regium (Wyville-Thomson, 1873). Zootaxa 4565, 201–212. doi: 10.11646/zootaxa.4565.2.4
Gan, Z., Yuan, J., Liu, X., Dong, D., Li, F., and Li, X. (2020). Comparative transcriptomic analysis of deep- and shallow-water barnacle species (Cirripedia, Poecilasmatidae) provides insights into deep-sea adaptation of sessile crustaceans. BMC Genomics 21:240. doi: 10.1186/s12864-020-6642-9
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. doi: 10.1093/sysbio/syq010
Hoek, P. P. C. (1883). Report on the Cirripedia collected by H.M.S. Challenger during the years 1873-76. Rep. Sei. Res. Challenger 8, 1–69.
Jones, D. S. (1993). A new Neolepas (Cirripedia: Thoracica: Scalpellidae) from an abyssal hydrothermal vent, southeast Pacific. Bull. Mar. Sci. 52, 937–948.
Jones, D. S. (2007). “The cirripedia of new caledonia,” in Compendium of Marine Species From New Caledonia, eds C. E. Payri and B. Richer de Forges (Noumea: Institut de Recherche pour le Développement), 289–294.
Jones, D. S. (2012). Australian barnacles (Cirripedia: Thoracica), distributions and biogeographical affinities. Integr. Comp Boil. 52, 366–387. doi: 10.1093/icb/ics100
Jones, D. S., and Hosie, A. M. (2016). A checklist of the barnacles (Cirripedia: Thoracica) of Singapore and neighboring waters. Raffles Bull. Zool. 34, 241–311.
Kolbasov, G. A., Chan, B. K., and Cheng, Y. R. (2017). Weltneria acanthostoma sp. nov., a burrowing barnacle (Cirripedia: Acrothoracica) from the deep-waters of the South China Sea. Zootaxa 4290, 591–599. doi: 10.11646/zootaxa.4290.3.12
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Kvile, K. Ø, Taranto, G. H., Pitcher, T. J., and Morato, T. (2014). A global assessment of seamount ecosystems knowledge using an ecosystem evaluation framework. Biol. Conserv. 173, 108–120. doi: 10.1016/j.biocon.2013.10.002
Lin, H. C., Høeg, J. T., Yusa, Y., and Chan, B. K. K. (2015). The origins and evolution of dwarf males and habitat use in thoracican barnacles. Mol. Phylogenet. Evol. 91, 1–11. doi: 10.1016/j.ympev.2015.04.026
Linse, K., Jackson, J. A., Fitzcharles, E., Sands, C. J., and Buckeridge, J. S. (2013). Phylogenetic position of Antarctic scalpelliformes (Crustacea: Cirripedia: Thoracica). Deep Sea Res. I 73, 99–116.
Lobo, J., and Tuaty-Guerra, M. (2017). A new deep-sea Cirripedia of the genus Heteralepas from the northeastern Atlantic. Eur. J. Taxon. 385, 1–14.
Miller, K. J., and Gunasekera, R. M. (2017). A comparison of genetic connectivity in two deep sea corals to examine whether seamounts are isolated islands or stepping stones for dispersal. Sci. Rep. 7:46103.
Mironov, A. N., and Krylova, E. M. (2006). “Origin of the fauna of the meteor seamounts, North-Eastern Atlantic,” in Biogeography of the North Atlantic Seamounts, eds A. N. Mironov, A. V. Gebruk, and A. J. Southward (Moscow: KMK Scientific Press), 22–57.
Moriarty, J. E., Sachs, J. A., and Jones, K. (2008). Direction locomotion in a turtle barnacle, Chelonibia testidunaria, on the green turtle, Chelonia mydas. Mar. Turtle Newslett. 119, 1–4.
Newman, W. A. (1979). A new scalpellid (Cirripedia): a Mesozoic relic living near an abyssal hydrothermal spring. Trans. San Diego Soc. Nat. Hist. 19, 153–167.
Newman, W. A. (1986). “Origin of the Hawaiian marine fauna: dispersal and vicarlance as indicated by barnacles and other organisms,” in Crustacean Biogeography, eds R. Gore and K. Heck (Rotterdam: Routledge), 21–49. doi: 10.1201/9781315140674-2
Newman, W. A., and Jones, W. J. (2011). Two Northeast Pacific deep-water barnacle populations (Cirripedia: Calanticidae and Pachylasmatidae) from seamounts of the Juan de Fuca Ridge; “insular” endemics stemming from Tethys, or by subsequent dispersal from the western Pacific center of distribution? Zootaxa 2789, 49–68. doi: 10.11646/zootaxa.2789.1.3
Ozaki, Y., Yusa, Y., Yamato, S., and Imaoka, T. (2008). Reproductive ecology of the pedunculated barnacle Scalpellum stearnsii (Cirripedia: Lepadomorpha: Scalpelidae). J. Mar. Biol. Assoc. U.K. 88, 77–83. doi: 10.1017/s0025315408000131
Pilsbry, H. A. (1907). The barnacles (Cirripedia) contained in the collection of the U. S. national museum. Bull. U.S. Natl. Museum 9, 1–122. doi: 10.5479/si.03629236.60.1
Poltarukha, O. P. (2011). On the barnacle (Cirripedia, Thoracica) fauna of the North Atlantic. Bull. Moscow Soc. Nat. 116, 31–39.
Poltarukha, O. P., and Mel’nik, V. F. (2012). New records of deep-sea barnacles (Cirripedia: Thoracica: Scalpelliformes) from the Clarion-Clipperton region, Pacific Ocean. Zootaxa 3297, 34–40. doi: 10.11646/zootaxa.3297.1.2
Poltarukha, O. P., and Zevina, G. B. (2006). “Barnacles (Cirripedia, Thoracica) of the Reykjanes Ridge,” in Biogeography of the North Atlantic Seamounts, eds A. N. Mironov, A. V. Gebruk, and A. J. Southward (Moscow: KMK Scientific Press), 152–161.
Posada, D., and Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818. doi: 10.1093/bioinformatics/14.9.817
Rao, L. M. V., and Newman, W. A. (1972). Thoracic cirripedia from guyots of the mid-pacific mountains. Trans. San Diego Soc. Nat. Hist. 17, 69–94. doi: 10.5962/bhl.part.19960
Rogers, A. D. (1994). The biology of seamounts. Adv. Mar. Biol. 30, 305–350. doi: 10.1016/s0065-2881(08)60065-6
Ronquist, F., Teslenko, M., Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MRBAYES 3.2: efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Shalaeva, K., and Boxshall, G. (2014). An illustrated catalogue of the scalpellid barnacles (Crustacea: Cirripedia: Scalpellidae) collected during the HMS “Challenger” expedition and deposited in the Natural History Museum, London. Zootaxa 3804, 1–63. doi: 10.11646/zootaxa.3804.1.1
Shalaeva, K., and Newman, W. A. (2016). Zevinaella—a new barnacle genus (Scalpellomorpha: Arcoscalpellinae) associated with crinoids (Echinodermata) from the Caribbean. Zootaxa 4072, 151–170. doi: 10.11646/zootaxa.4072.2.1
Shank, T. M. (2010). Seamounts: deep-ocean laboratories of faunal connectivity, evolution, and endemism. Oceanography 23, 108–122. doi: 10.5670/oceanog.2010.65
Southward, A. J. (2005). Systematics and ecology of a new species of stalked barnacle (Cirripedia: Thoracica: Scalpellomorpha: Eolepadidae: Neolepadini) from the Pacific-Antarctic Ridge at 38°S. Senckenbergiana Maritime 35, 147–156. doi: 10.1007/bf03043683
Southward, A. J., and Jones, D. S. (2003). A revision of stalked barnacles (Cirripedia: Thoracica: Scalpellomorpha: Eolepadidae: Neolepadinae) associated with hydrothermalism, including a description of a new genus and species from a volcanic seamount off Papua New Guinea. Senckenbergiana Maritime 32, 77–93. doi: 10.1007/bf03043086
Stocks, K. (2004). “Seamount Invertebrates: Composition and Vulnerability to Fishing,” in Seamounts: Biodiversity and Fisheries, eds T. Morato and D. Pauly (Vancouver: Fisheries Centre, University of British Columbia), 17–34.
Tunnicliffe, V., and Southward, A. J. (2004). Growth and breeding of a primitive stalked barnacle Leucolepas longa (Cirripedia: Scalpellomorpha: Eolepadidae: Neolepadinae) inhabiting a volcanic seamount off Papua New Guinea. J. Mar. Biol. Assoc. U.K. 84, 121–132. doi: 10.1017/s0025315404008987h
Wilson, R. T. J., and Kaufmann, R. S. (1987). “Seamount Biota and Biogeography,” in Seamounts, Islands, and Atolls, Geophysical Monograph Series, ed. B. H. Keating (Washington: American Geophysical Union), 355–377. doi: 10.1029/gm043p0355
Worm, B., Lotze, H. K., and Myers, R. A. (2003). Predator diversity hotspots in the blue ocean. Proc. Natl. Acad. Sci. U.S.A. 100, 9884–9888. doi: 10.1073/pnas.1333941100
Yamaguchi, T., Newman, W. A., and Hashimoto, J. (2004). A cold seep barnacle (Cirripedia: Neolepadinae) from Japan and the age of the vent/seep fauna. J. Mar. Biol. Assoc. U.K. 84, 111–120. doi: 10.1017/s0025315404008975h
Young, P. S. (1998a). Cirripeds (Crustacea) from the Mid-Atlantic Ocean Ridge collected by the submersible Nautile. Cah. Biol. mar. 39, 109–119.
Young, P. S. (1998b). The Cirripedia (Crustacea) collected by the fisheries steamer meteor in the eastern Atlantic. Arquivos Museu Nacional Rio Janeiro 58, 1–54.
Young, P. S. (1999). The Cirripedia (Crustacea) collected by the RV Marion Dufresne along the Vitória-Trindade seamounts (Brazil). Zoosystema 21, 607–624.
Young, P. S. (2001). Deep-sea Cirripedia Thoracica (Crustacea) from the northeastern Atlantic collected by French expeditions. Zoosystema 23, 705–756.
Young, P. S. (2002). The Verrucidae (Crustacea, Cirripedia) from the western coast of North America, with a revision on the genus Altiverruca. Arq. Mus. Nac. 60, 5–40.
Yusa, Y., Yasuda, N., Yamamoto, T., Watanabe, H. K., Higashiji, T., Kaneko, A., et al. (2018). Direct growth measurements of two deep-sea scalpellid barnacles, Scalpellum stearnsii and Graviscalpellum pedunculatum. Zool. Stud. 57:29.
Zevina, G. B. (1975). Cirripedia thoracica of the American Mediterranean. Trudy Instit. Okeanol. Akademii Nauk SSSR 100, 233–258.
Zevina, G. B. (1978). A new system of the family Scalpellidae pilsbry (Cirripedia, Thoracica). 2. Subfamilies Arcoscallpellinae and Meroscalpellinae. Zool. Zh. 57, 1343–1352.
Zevina, G. B. (1981a). “Barnacles (Cirripedia),” in Benthos of the Submarine Mountains Marcus-Necker and Adjacent Pacific Regions, ed. A. P. Kuznecov (Moscow: Institut Okeanologii), 56–62.
Zevina, G. B. (1981b). Barnacles of the Suborder Lepadomorpha (Cirripedia, Thorocica) of the World Ocean, Part 1 Family Scalpellidae. Leningrad: Opredeliteli po Faune SSSR.
Zevina, G. B. (1982). Barnacles of the suborder Lepadomorpha (Cirripedia, Thoracica) of the World Ocean, Part II. Leningrad: Opredeliteli po Faune SSSR.
Zevina, G. B. (1983). The Cirripedia from peaks of the Nasca Ridge mountains (Pacific Ocean). Zool. Zh. 62, 1635–1642.
Keywords: barnacle, Arcoscalpellum, Glyptelasma, seamount, integrative taxonomy
Citation: Gan Z, Xu P, Li X and Wang C (2020) Integrative Taxonomy Reveals Two New Species of Stalked Barnacle (Cirripedia, Thoracica) From Seamounts of the Western Pacific With a Review of Barnacles Distributed in Seamounts Worldwide. Front. Mar. Sci. 7:582225. doi: 10.3389/fmars.2020.582225
Received: 11 July 2020; Accepted: 25 August 2020;
Published: 25 September 2020.
Edited by:
Tito Monteiro da Cruz Lotufo, University of São Paulo, BrazilCopyright © 2020 Gan, Xu, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinzheng Li, lixzh@qdio.ac.cn; Chunsheng Wang, wangsio@sio.org.cn; Zhibin Gan, ganzhibin@qdio.ac.cn
†These authors have contributed equally to this work
 Zhibin Gan
Zhibin Gan Peng Xu
Peng Xu Xinzheng Li
Xinzheng Li Chunsheng Wang3,6*
Chunsheng Wang3,6*