Abstract
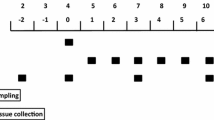
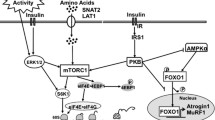
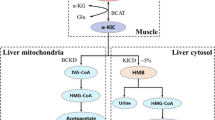
When neonatal pigs continuously fed formula are supplemented with leucine pulses, muscle protein synthesis and body weight gain are enhanced. To identify the responsible mechanisms, we combined plasma metabolomic analysis with transcriptome expression of the transcriptome and protein catabolic pathways in skeletal muscle. Piglets (n = 23, 7-day-old) were fed continuously a milk replacement formula via orogastric tube for 21 days with an additional parenteral infusion (800 μmol kg−1 h−1) of either leucine (LEU) or alanine (CON) for 1 h every 4 h. Plasma metabolites were measured by liquid chromatography-mass spectrometry. Gene and protein expression analyses of longissimus dorsi muscle were performed by RNA-seq and Western blot, respectively. Compared with CON, LEU pigs had increased plasma levels of leucine-derived metabolites, including 4-methyl-2-oxopentanoate, beta-hydroxyisovalerate, β-hydroxyisovalerylcarnitine, and 3-methylglutaconate (P ≤ 0.05). Leucine pulses downregulated transcripts enriched in the Kyoto Encyclopedia of Genes and Genomes terms “spliceosome,” “GAP junction,” “endocytosis,” “ECM-receptor interaction,” and “DNA replication”. Significant correlations were identified between metabolites derived from leucine catabolism and muscle genes involved in protein degradation, transcription and translation, and muscle maintenance and development (P ≤ 0.05). Further, leucine pulses decreased protein expression of autophagic markers and serine/threonine kinase 4, involved in muscle atrophy (P ≤ 0.01). In conclusion, results from our studies support the notion that leucine pulses during continuous enteral feeding enhance muscle mass gain in neonatal pigs by increasing protein synthetic activity and downregulating protein catabolic pathways through concerted responses in the transcriptome and metabolome.





Similar content being viewed by others
Data availability
Raw data are provided as online resources.
Abbreviations
- AA:
-
Amino acids
- ABLIM3:
-
Actin binding LIM protein family member 3
- ALDH4A1:
-
Aldehyde dehydrogenase 4 family member A1
- AMPKα:
-
AMP-activated protein kinase α
- ANKH:
-
ANKH inorganic pyrophosphate transport regulator
- ANKRD6:
-
Ankyrin repeat domain 6
- AP4S1:
-
Adaptor related protein complex 4 sigma 1 subunit
- ASF1B:
-
Anti-silencing function 1B histone chaperone
- AU:
-
Arbitrary units
- BCAA:
-
Branched-chain amino acids
- BCL-XL:
-
B-cell lymphoma-extra large
- BW:
-
Body weight
- CCNJL:
-
Cyclin J like
- CD9:
-
CD9 molecule
- CDCA2:
-
Cell division cycle associated 2
- CHRNA3:
-
Cholinergic receptor nicotinic alpha 3 subunit
- CON:
-
Control group
- DAVID:
-
Database for Annotation, Visualization, and Integrated Discovery
- DEGs:
-
Differentially expressed genes
- DSCC1:
-
DNA replication and sister chromatid cohesion 1
- EAA:
-
Essential amino acids
- ERLEC1:
-
Endoplasmic reticulum lectin 1
- ESF1:
-
ESF1 nucleolar pre-rRNA processing protein, homolog
- FN1:
-
Fibronectin 1
- FOXO:
-
Forkhead box protein O
- GNRHR2:
-
Gonadotropin-releasing hormone II receptor
- GO:
-
Gene ontology
- GPR37L1:
-
G protein-coupled receptor 37 like 1
- GRM7:
-
Glutamate metabotropic receptor 7
- HAUS1:
-
HAUS augmin like complex subunit 1
- HMGCLL1:
-
3-Hydroxymethyl-3-methylglutaryl-CoA lyase like 1
- INO80:
-
Chromatin-remodeling ATPase INO80
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- KIC:
-
α-Ketoisocaproic acid
- KLHDC3:
-
Kelch domain containing 3
- LC3I/II:
-
Microtubule-associated protein 1A/1B light chain 3A
- LD:
-
Longissimus dorsi
- LEU:
-
Leucine supplemented group
- LPXN:
-
Leupaxin
- MAFbx:
-
Muscle Atrophy F-Box/Atrogin-1
- MFAP3L:
-
Microfibrillar associated protein 3 like
- MMP15:
-
Matrix metallopeptidase 15
- mTORC1:
-
Mammalian target of rapamycin complex 1
- MuRF-1:
-
Muscle RING-finger protein-1
- NUAK2:
-
NUAK family SNF1-like kinase 2
- OFD1:
-
Oral-facial-digital syndrome 1 protein
- OPHN1:
-
Oligophrenin 1
- OPTN:
-
Optineurin
- P/T:
-
Phosphorylated/total protein
- PARP8:
-
Protein mono-ADP-ribosyltransferase
- PC:
-
Principal component analysis
- PDE10A:
-
Phosphodiesterase 10A
- PKB/Akt:
-
Protein kinase B
- POPDC3:
-
Popeye domain containing 3
- REPS2:
-
RalBP1-associated Eps domain-containing 2
- RGS3:
-
Regulator of G-protein signaling 3
- RNA-seq:
-
High-throughput RNA sequencing
- RNF17:
-
Ring finger protein 17
- ROMO1:
-
Reactive oxygen species modulator 1
- RPKM:
-
Reads per kilobase per million mapped reads
- SERBP1:
-
Plasminogen activator inhibitor 1 RNA-binding protein
- SF3B4:
-
Splicing factor 3b subunit 4
- SIRT-1:
-
Sirtuin 1
- SNX32:
-
Sorting nexin 32
- STK4:
-
Serine/threonine kinase 4
- TAS2R40:
-
Taste 2 receptor member 40
- TBX21:
-
T-box 21
- TMEM117:
-
Transmembrane protein 117
- UBL4A:
-
Ubiquitin like 4A
- VNN2:
-
Vanin 2
- ZBTB32:
-
Zinc finger and BTB domain containing 32
- ZNF207:
-
Zinc finger protein 207
- ZNF385D:
-
Zinc finger protein 385D
- ZNF652:
-
Zinc finger protein 652
References
Anufrieva KS, Shender VO, Arapidi GP, Pavlyukov MS, Shakhparonov MI, Shnaider PV, Butenko I, Lagarkova MA, Govorun VM (2018) Therapy-induced stress response is associated with downregulation of pre-mRNA splicing in cancer cells. Genome Med 10(1):49
Aynsley-Green A, Adrian TE, Bloom SR (1982) Feeding and the development of enteroinsular hormone secretion in the preterm infant: effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatr Scand 71(3):379–383
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300
Børsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR (2008) Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr 27(2):189–195
Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA (2013) Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab 305(5):E620–E631
Boutry C, El-Kadi SW, Suryawan A, Steinhoff-Wagner J, Stoll B, Orellana RA, Nguyen HV, Kimball SR, Fiorotto ML, Davis TA (2016) Pulsatile delivery of a leucine supplement during long-term continuous enteral feeding enhances lean growth in term neonatal pigs. Am J Physiol Endocrinol Metab 310(8):E699–E713
Braegger C, Decsi T, Dias JA, Hartman C, Kolacek S, Koletzko B, Koletzko S, Mihatsch W, Moreno L, Puntis J, Shamir R, Szajewska H, Turck D, van Goudoever J, ESPGHAN Committee on Nutrition (2010) Practical approach to pediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 51(1):110–122
Bui T, Rennhack J, Mok S, Ling C, Perez M, Roccamo J, Andrechek ER, Moraes C, Muller WJ (2019) Functional redundancy between β1 and β3 integrin in activating the IR/Akt/mTORC1 signaling axis to promote ErbB2-driven breast cancer. Cell Rep 29(3):589–602.e6
Chen Scarabelli C, McCauley RB, Yuan Z, Di Rezze J, Patel D, Putt J, Raddino R, Allebban Z, Abboud J, Scarabelli GM, Chilukuri K, Gardin J, Saravolatz L, Faggian G, Mazzucco A, Scarabelli TM (2008) Oral administration of amino acidic supplements improves protein and energy profiles in skeletal muscle of aged rats: elongation of functional performance and acceleration of mitochondrial recovery in adenosine triphosphate after exhaustive exertion. Am J Cardiol 101(11A):42E–48E
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Davis TA, Burrin DG, Fiorotto ML, Nguyen HV (1996) Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am J Physiol 270(5 Pt 1):E802–E809
Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR (2000) Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279:E1226–E1234
de Ville K, Knapp E, Al-Tawil Y, Berseth CL (1998) Slow infusion feedings enhance duodenal motor responses and gastric emptying in preterm infants. Am J Clin Nutr 68:103–108
Dziechciarz P, Horvath A, Shamir R, Szajewska H (2007) Meta-analysis: enteral nutrition in active Crohn's disease in children. Aliment Pharmacol Ther 26(6):795–806
El-Kadi SW, Suryawan A, Gazzaneo MC, Srivastava N, Orellana RA, Nguyen HV, Lobley GE, Davis TA (2012) Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab 302(6):E674–E686
El-Kadi SW, Boutry C, Suryawan A, Gazzaneo MC, Orellana RA, Srivastava N, Nguyen HV, Kimball SR, Fiorotto ML, Davis TA (2018) Intermittent bolus feeding promotes greater lean growth than continuous feeding in a neonatal piglet model. Am J Clin Nutr 108(4):830–841
Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA (2005) Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288:E914–E921
Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA (2010) Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 140(8):1418–1424
Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, Dai H, Stewart SJ, DeHaven CD, Miller LAD (2014) High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 4:132
Goc A, Choudhary M, Byzova TV, Somanath PR (2011) TGFβ- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR). J Cell Physiol 226(11):3004–3013
Grant J, Denne SC (1991) Effect of intermittent versus continuous enteral feeding on energy expenditure in premature infants. J Pediatr 118:928–932
Harper AE, Benevenga NJ, Wohlhueter RM (1970) Effects of ingestion disproportionate amounts of amino acids. Physiol Rev 50:428–558
Harrell FE, Dupont C (2019) Hmisc: Harrell Miscellaneous. R package version 4.2-0. https://CRAN.R-project.org/package=Hmisc. Accessed 13 Jan 2020
Hastie T, Tibshirani R, Narasimhan B, Chu G (2019) Imputation for microarray data. R package version 1.58.0. https://www.bioconductor.org/packages/release/bioc/html/impute.html. Accessed 13 Jan 2020
Hill DJ, Cameron DJ, Francis DE, Gonzalez-Andaya AM, Hosking CS (1995) Challenge confirmation of late-onset reactions to extensively hydrolyzed formulas in infants with multiple food protein intolerance. J Allergy Clin Immunol 96(3):386–394
Hyttinen JM, Niittykoski M, Salminen A, Kaarniranta K (2013) Maturation of autophagosomes and endosomes: a key role for Rab7. Biochim Biophys Acta 3:503–510
Jeffares DC, Penkett CJ, Bähler J (2008) Rapidly regulated genes are intron poor. Trends Genet 24:375–378
Kainulainen H, Hulmi JJ, Kujala UM (2013) Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc Sport Sci Rev 41(4):194–200
Kao M, Columbus DA, Suryawan A, Steinhoff-Wagner J, Hernandez-Garcia A, Nguyen HV, Fiorotto ML, Davis TA (2016) Enteral β-hydroxy-β-methylbutyrate supplementation increases protein synthesis in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 310(11):E1072–E1084
Kim SW, Wu G (2004) Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 134:625–630
Kittler R, Putz G, Pelletier L, Poser I, Heninger AK, Drechsel D, Fischer S, Konstantinova I, Habermann B, Grabner H, Yaspo ML, Himmelbauer H, Korn B, Neugebauer K, Pisabarro MT, Buchholz F (2004) An endoribonuclease-prepared siRNA screen in human cells identifies genes essential for cell division. Nature 432(7020):1036–1040
Kleinridders A, Pogoda HM, Irlenbusch S, Smyth N, Koncz C, Hammerschmidt M, Brüning JC (2009) PLRG1 is an essential regulator of cell proliferation and apoptosis during vertebrate development and tissue homeostasis. Mol Cell Biol 29(11):3173–3185
Kohlmeier M (2015) Nutrient metabolism: structures, functions, and genes. Academic Press, London
Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH (2007) AMP-activated protein kinase agonists increase mRNA content of the muscle specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab 292:E1555–E1567
Lessard SJ, Rivas DA, So K, Koh HJ, Queiroz AL, Hirshman MF, Fielding RA, Goodyear LJ (2016) The AMPK-related kinase SNARK regulates muscle mass and myocyte survival. J Clin Investig 126(2):560–570
Manjarín R, Columbus DA, Suryawan A, Nguyen HV, Hernandez-García AD, Hoang NM, Fiorotto ML, Davis T (2016) Leucine supplementation of a chronically restricted protein and energy diet enhances mTOR pathway activation but not muscle protein synthesis in neonatal pigs. Amino Acids 48(1):257–267
Manjarín R, Columbus DA, Solis J, Hernandez-García AD, Suryawan A, Nguyen HV, McGuckin MM, Jimenez RT, Fiorotto ML, Davis TA (2018) Short- and long-term effects of leucine and branched-chain amino acid supplementation of a protein- and energy-reduced diet on muscle protein metabolism in neonatal pigs. Amino Acids 50(7):943–959
Mashako MN, Bernard C, Cezard JP, Chayvialle JA, Navarro J (1987) Effect of total parenteral nutrition, constant rate enteral nutrition, and discontinuous oral feeding on plasma cholecystokinin immunoreactivity in children. J Pediatr Gastroenterol Nutr 6(6):948–952
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
National Research Council (1998) Nutrient Requirements of Swine: 10th Revised Edition. The National Academies Press, Washington, DC
Pandya-Jones A (2011) Pre-mRNA splicing during transcription in the mammalian system. Wiley Interdiscip Rev RNA 2(5):700–717
Parker P, Stroop S, Greene H (1981) A controlled comparison of continuous versus intermittent feeding in the treatment of infants with intestinal disease. J Pediatr 99(3):360–364
Paulsen RD, Soni DV, Wollman R, Hahn AT, Yee MC, Guan A, Hesley JA, Miller SC, Cromwell EF, Solow-Cordero DE, Meyer T, Cimprich KA (2009) A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell 35(2):228–239
Piepho HP (2009) Data transformation in statistical analysis of field trials with changing treatment variance. Agron J 101:865–869
Sandri M (2010) Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol 298(6):C1291–C1297
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504
Shulman RJ, Redel CA, Stathos TH (1994) Bolus versus continuous feedings stimulate small-intestinal growth and development in the newborn pig. J Pediatr Gastroenterol Nutr 18(3):350–354
Singh J, Padgett RA (2009) Rates of in situ transcription and splicing in large human genes. Nat Struct Mol Biol 16:1128–1133
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Suryawan A, Rudar M, Fiorotto ML, Davis TA (2020) Differential regulation of mTORC1 activation by leucine and β-hydroxy-β-methylbutyrate in skeletal muscle of neonatal pigs. J Appl Physiol 128(2):286–295
Tannous RE, Rogers QR, Harper AE (1963) Effect of leucine-isoleucine and valine antagonism on the pattern of free amino acids in blood plasma and several tissues of the rat. Fed Proc 22:202–210
The UniProt Consortium (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515
Tooze SA, Abada A, Elazar Z (2014) Endocytosis and autophagy: exploitation or cooperation? Cold Spring Harb Perspect Biol 6(5):a018358
Torrazza RM, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA (2010) Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140(12):2145–2152
Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB (2001) Missing value estimation methods for DNA microarrays. Bioinformatics 17(6):520–525
Wei B, Dui W, Liu D, Xing Y, Yuan Z, Ji G (2013) MST1, a key player, in enhancing fast skeletal muscle atrophy. BMC Biol 11:12
Wheatley SM, El-Kadi SW, Suryawan A, Boutry C, Orellana RA, Nguyen HV, Davis SR, Davis TA (2014) Protein synthesis in skeletal muscle of neonatal pigs is enhanced by administration of β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab 306:E91–E99
Wickramasinghe S, Rincon G, Islas-Trejo A, Medrano JF (2012) Transcriptional profiling of bovine milk using RNA sequencing. BMC Genomics 13:45
Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA (2010) Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140:264–270
Yoshii SR, Mizushima N (2017) Monitoring and measuring autophagy. Int J Mol Sci 18(9):E1865
Author information
Authors and Affiliations
Contributions
Conceptualization—CBR, MF and TD. Data curation—CBR, AS and HN. Formal analysis—RM, BP, AC, MM, and MI. Funding acquisition—MF and TD. Investigation—CBR, AS, HN, MF and TD. Methodology—CBR, AS and TD. Project administration—MF and TD. Resources—MF and TD. Supervision—MF and TD. Validation—MF and TD. Visualization—RM, BP, AC, MM, and MI. Writing—original draft—RM, BP, AC, MM, and MI. Writing—review and editing—all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Experiments were carried out in accordance with the Institutional Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (2011).
Additional information
Handling Editor: G. Wu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
726_2020_2894_MOESM1_ESM.xlsx
Online Resource 1 Metabolomic analysis results in plasma from neonatal pigs fed continuously with an orogastric tube for 21 days and supplemented with either leucine or alanine pulses parenterally at 4-h intervals (XLSX 180 kb)
726_2020_2894_MOESM2_ESM.xlsx
Online Resource 2 Differentially expressed genes (P ≤ 0.05) in skeletal muscle tissue from neonatal pigs fed continuously with an orogastric tube for 21 days and supplemented with either leucine or alanine pulses parenterally at 4-h intervals (XLSX 113 kb)
Rights and permissions
About this article
Cite this article
Manjarín, R., Boutry-Regard, C., Suryawan, A. et al. Intermittent leucine pulses during continuous feeding alters novel components involved in skeletal muscle growth of neonatal pigs. Amino Acids 52, 1319–1335 (2020). https://doi.org/10.1007/s00726-020-02894-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02894-5