Abstract—
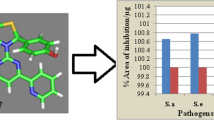
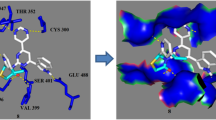
The recent study reported the designing of substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives and assessed computationally to calculate the bioactivity and physicochemical properties. The substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives represented the bioactivity score in the zone for an active drug molecule and were in compliance with the Lipinski Rule of five. Then the synthesis, characterization, and biological screening as antimicrobial potential and percent viability of cells were carried out for the substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives. The zone of inhibition and minimum inhibitory concentration (MIC) findings portrayed that the compounds-(IV) and compound-(V) possessed better antimicrobial activity than the reference drug ciprofloxacin, while the significant antimicrobial potential was observed by other members of the series. The molecular docking studies were performed to assist the in vitro antimicrobial results and the findings exhibited that significant H-bonding in between the substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives and the residues of GlcN-6-P-synthase, like ASP 474 (I–IX), SER 316 (I–VI), ASN 522 (I–IX), TRP 313 (V) with good binding affinity ranging –7.7 to –6.8 kcal/mole. The compounds represented the less toxic effects to the HepG2 cells and the percent viability of the cells ranging from 93–98%, 73–78% and 70–76% up to 3.125, 50 , 100 mmol/L respectively.






Similar content being viewed by others
REFERENCES
Babu, K.R., Eeshwaraiah, B., Aravind, D., Harshadas, M.M., Rallabaldi, M.R., Apurba, B., and Rakeshwar, B., Monatsh Chem., 2008, vol. 139, pp. 179–181. https://doi.org/10.1007/s00706-007-0772-5
Kulandaivelu, U., Padmini, V.G., Suneetha, K., Shireesha, B., Vidyasagar, J.V., Rao, T.R., Jayaveera, K.N., Basu, A., and Jayaprakash, V., Arch. Pharm., 2011, vol. 344, pp. 84–90. https://doi.org/10.1002/ardp.201000201
Rohit, K., Shabana, I.K., Aparna, B., Mohit, T., and Diwan, S.R., Eur. J. Med. Chem., 2017, vol. 131, pp. 126–140. https://doi.org/10.1016/j.ejmech.2017.03.007
Hanwen, W., Fei, M., Shuaishuai, N., Feifei, C., Baoli, L., Xiaoxia, Q., Linghao, H., Manjiong, W., Xinyu, Z., Jin, Z., Lefu, L., and Jian, L., Eur. J. Med. Chem., 2018, vol. 145, pp. 235–251. https://doi.org/10.1016/j.ejmech.2017.12.090
Leite, A.C.L., da Silva, K.P., de Souza, I.A., de Araujo, J.M., and Brondali, D.J., Eur. J. Med. Chem., 2004, vol. 39, pp. 1059–1065. https://doi.org/10.1016/j.ejmech.2004.09.007
Prasanthi, G., Prasad, K.V.S.R.G., and Bharathi, K., Eur. J. Med. Chem., 2013, vol. 66, pp. 516–525. https://doi.org/10.1016/j.ejmech.2013.06.006
Moreira Leal, C., Lopes Pereira, S., Kummerle, A.E., Moreira Leal, D., Tesch, R., de Sant'Anna, C.M.R., Fraga, C.A.M., Barreiro, E.J., Takashi Sudo, R., Zapata-Sudo, G., Eur. J. Med. Chem., 2012, vol. 55, pp. 49–57. https://doi.org/10.1016/j.ejmech.2012.06.056
Arshad, M., Bhat, A.R., Pokharel, S., Ki,m, J-E., Lee, E.J., Athar, F., and Choi, I., Eur. J. Med. Chem., 2014, vol. 71, pp. 229–236. https://doi.org/10.1016/j.ejmech.2013.11.008
Wani, M.Y., Bhat, A.R., Azam, A., Choi, I., and Athar, F., Eur. J. Med. Chem., 2012, vol. 48, pp. 313–320. https://doi.org/10.1016/j.ejmech.2011.12.033
Chavarria, D., Fernandes, C., Silva, V., Silva, C., Gil-Martins, E., Soares, P., Silva, T., Silva, R., Remião, F., Oliveira, P. J., and Borges, F., Eur. J. Med. Chem., 2020, vol. 185, p. 111 770. https://doi.org/10.1016/j.ejmech.2019.111770
Singh, I.P., Jain, S.K., and Kour, A., Eur. J. Med. Chem., 2010, vol. 4, p. 33.
da Silva Ferreira, W., Freire-de-Lima, L., Barbosa Saraiva, V., Alisson-Silva, F., Mendonca-Previato, L., Previato, J.O., Echevarria, A., and Freire de Lima, M.E. Bioorg. Med. Chem., 2008, vol. 16, pp. 2984–2991.
Gupta, S.D., Rao, G.B., Bommaka, M.K., Raghavendra, N.M., Aleti, S., Arab. J. Chem., 2016, vol. 9, pp. 1878–5352. https://doi.org/10.1016/j.arabjc.2014.08.004
Wang, S., Bao, L., Song, D., Wang, J., and Cao, X., Bioorg. Med. Chem. Lett., 2019, vol. 29, p. 126 661. https://doi.org/10.1016/j.bmcl.2019.126661
Brum, J.O.C., Neto, D.C.F., de Almeida, J.S.F.D., Lima, J.A., Kuca, K., França, T.C.C., and Figueroa-Villar, J.D. Int. J. Mol. Sci., 2019, vol. 20, p. 3944. https://doi.org/10.3390/ijms20163944
Arshad, M., J. Iran Chem. Soc., 2020, vol. 17, pp. 1305–1315. https://doi.org/10.1007/s13738-020-01855-9
Ghoneim, A.A., El-Farargy, A.F., Elkanzi, N.A.A. J. Iran Chem. Soc., 2019, vol. 16, pp. 319–325. https://doi.org/10.1007/s13738-019-01768-2
Hamid, A.M.A., Shehta, W., J. Iran Chem. Soc., 2018, vol. 15, pp. 2771–2779. https://doi.org/10.1007/s13738-018-1464-2
Bhosle, M.R., Andil, P., Wahul, D., Bondle, G.M., Sarkate, A., Tiwari, S.V., J Iran Chem Soc., 2019, vol. 16, pp. 1553–1561. https://doi.org/10.1007/s13738-019-01633-2
Ślifirski, G., Król, M., Kleps, J., Podsadni, P., Belka, M., Bączek, T., Siwek, A., Stachowicz, K., Szewczyk, B., Nowak, G., Bojarski, A., Kozioł, A. E., Turło, J., Herold, F., Eur. J. Med. Chem., 2019, vol. 180, pp. 383–397. https://doi.org/10.1016/j.ejmech.2019.07.027
da S. Falcao, E.P., de Melo, S.J., Srivastava, R.M., de A. Catanho, M.T.J., and Do Nascimento, S. C., Eur. J. Med. Chem., 2006, vol. 41, pp. 276–282. https://doi.org/10.1016/j.ejmech.2005.09.009
Kotaiah, Y., Nagaraju, K., Harikrishna, N., Venkata Rao, C., Yamini, L., Vijjulatha, M., Eur. J. Med. Chem., 2014, vol. 75, pp. 195–202. https://doi.org/10.1016/j.ejmech.2014.01.006
Kaur, H., Balzarini, J., Kock, C. de., Smith, P.J., Chibale, K., Singh, K., Eur. J. Med. Chem., 2015, vol. 101, pp. 52–62. https://doi.org/10.1016/j.ejmech.2015.06.024
Maurya, S.S., Bahuguna, A., Khan, S.I., Kumar, D., Kholiya, R., Rawat, D.S., Eur. J. Med. Chem., 2019, vol. 162, pp. 277–289. https://doi.org/10.1016/j.ejmech.2018.11.021
Lee, H.W., Kim, B.Y., Ahn, J.B., Kang, S.K., Lee, J.H., Shin, J.S., Ahn, S.K., Lee, S.J., Yoon, S.S., Eur. J. Med. Chem., 2005, vol. 40, pp. 862–874. https://doi.org/10.1016/j.ejmech.2005.03.019
Verbitskiy, E.V., Cheprakova, E.M., Slepukhin, P.A., Kravchenko, M.A. Skornyakov, S.N., Rusinov, G.L., Chupakhin, O.N., Charushin, V.N., Eur. J. Med. Chem., 2015, vol. 97, pp. 225–234. https://doi.org/10.1016/j.ejmech.2015.05.007
Hafez, H.N., Hussein, H.A.R., El-Gazzar, Abdel-Rahman B.A., Eur. J. Med. Chem., 2010, vol. 45, pp. 4026–4034. https://doi.org/10.1016/j.ejmech.2010.05.060
Zhu, M., Ma, L., Zhou, H., Dong, B., Wang, Y., Wang, Z., Zhou, J., Zhang, G., Wang, J., Liang, C., Cen, S., Wang, Y., Eur. J. Med. Chem., 2020, vol. 185, p. 111 866. https://doi.org/10.1016/j.ejmech.2019.111866
Keri, R. S., Hosamani, K. M., Shingalapur, R. V., Hugar, M. H., Eur. J. Med. Chem., 2010, vol. 45, pp. 2597–2605. https://doi.org/10.1016/j.ejmech.2010.02.048
Jin, X., Merrett, J., Tong, S., Flower, B., Xie, J., Yu, R., Tian, S., Gao, L., Zhao, J., Wang, X., Jiang, Tao, Proud, C. G., Eur. J. Med. Chem., 2019, vol. 162, pp. 735–751. https://doi.org/10.1016/j.ejmech.2018.10.070
Shehab, W.S., El-Shwiniy, W.H., J. Iran. Chem. Soc., 2018, vol. 15, p. 431. https://doi.org/10.1007/s13738-017-1244-4
Arshad, M., Khan, M. S., Nami, S. A. A., Ahmad, D., Rus. J. Gen. Chem., 2018, vol. 88, pp. 2154–2162. https://doi.org/10.1134/S1070363218100213
Carradori, S., Bizzarri, B., D’Ascenzio, M., Monte, C. D., Grande, R., Rivanera, D., Zicari, A., Mari, E., Sabatino, M., Patsilinakos, A., Rino Ragno, R., and Daniela Secci, D., Eur. J. Med. Chem., 2017, vol. 140, pp. 274–292. https://doi.org/10.1016/j.ejmech.2017.09.026
Pejović, A., Denić, M.S., Stevanović, D., Damljanović, I., Vukićević, M., Kostova, K., Tavlinova-Kirilova, M., Randjelović, P., Stojanović, N.M., Bogdanović, G.A., Blagojević, P., D’hooghe, M., Radulović, N.S., and Vukićević, R.D., Eur. J. Med. Chem., 2014, vol. 83, pp. 57–73. https://doi.org/10.1016/j.ejmech.2014.05.062
Koppireddi, S., Komsani, J.R., Avula, S., Pombala, S., Vasamsetti, S., Kotamraju, S., and Yadla, R., Eur. J. Med. Chem., 2013, vol. 66, pp. 305–313. https://doi.org/10.1016/j.ejmech.2013.06.005
Rawal, R.K., Tripathi, R., Katti, S.B., Pannecouque, C., and De Clercq, E., Eur. J. Med. Chem., 2008, vol. 43, pp. 2800–2806. https://doi.org/10.1016/j.ejmech.2007.12.015
Raza, S., Srivastava, S.P., Srivastava, D.S., Srivastava, A.K., Haq, W., and Katti, S.B., Eur. J. Med. Chem., vol. 63, 2013, pp. 611–620. https://doi.org/10.1016/j.ejmech.2013.01.054
Revelant, G., Huber-Villaume, S., Dunand, S., Kirsch, G., Schohn, H., and Hesse, S., Eur. J. Med. Chem., 2015, vol. 94, pp. 102–112. https://doi.org/10.1016/j.ejmech.2015.02.053
Barbosa, V.A., Baréa, P., Mazia, R.S., Ueda-Nakamura, T., da Costa, W.F., Foglio, M.A., Ruiz, A.L.T.G., de Carvalho, J.E., Vendramini-Costa, D.B., Nakamura, C., and Sarragiotto, M.H., Eur. J. Med. Chem., 2016, vol. 124, pp. 1093–1104. https://doi.org/10.1016/j.ejmech.2016.10.018
Trotsko, N., Golus, J., Kazimierczak, P., Paneth, A., Przekora, A., Ginalska, G., and Wujec, M., Eur. J. Med. Chem., 2020, vol. 189, p. 112 045. https://doi.org/10.1016/j.ejmech.2020.112045
da Silva, D.S., da Silva, C.E.H., Soares, M.S.P., Juliana Hofstatter Azambuja, de Carvalho, T.R., Zimmer, G.C., Frizzo, C.P., Braganhol, E., Spanevello, R.M., and Cunico, W., Eur. J. Med. Chem., 2016, vol. 124, pp. 574–582. https://doi.org/10.1016/j.ejmech.2016.08.057
Alodeani, E.A., Arshad, M., and Izhari, M.A. Asian Pac.J. Health Sci. 2015, vol. 2, pp. 41–47. https://www.apjhs.com/pdf/8-Antileishmanial-screening-physicochemical-properties-and-drug-likeness-of-pyrazole-carbaldehyde-derivatives.pdf.
Alodeani, E.A., Arshad, M., and Izhari, M.A., Eur. J. Pharm. Med. Res., 2015, vol. 2, pp. 324–328. http://www.ejpmr.com/admin/assets/article_issue/ 1448880734.pdf.
Alodeani, E.A., Arshad, M., and Izhari, M.A. Asian Pac. J. Trop. Biomed., 2015, vol. 5, pp. 676–683. https://doi.org/10.1016/j.apjtb.2015.04.010
Alodeani, E.A., Arshad, M., and Izhari, M.A. Eur. J. Pharm. Med. Res., 2015, vol. 2, pp. 296–301. http://www.ejpmr.com/admin/assets/article_issue/ 1446625932.pdf.
Arshad, M. and Shadab, M., Eur. J. Pharm. Med. Res., 2017, vol. 4, pp. 364–368. http://www.ejpmr.com/ home/abstract_id/2154.
Arshad, M. and Shadab, M., Eur. J. Pharm. Med. Res. 2017, vol. 4, pp. 447–454. https://www.ejpmr.com/home/abstract_id/2267.
Arshad, M., Eur. J. Pharm. Med. Res., 2017, vol. 4, pp. 511–517. http://www.ejpmr.com/admin/assets/article_issue/1512459098.pdf.
Arshad, M., Russ. J. Gen. Chem., 2018, vol. 88, pp. 1886–1891. https://doi.org/10.1134/S1070363218090207
Arshad, M., Khan, M.S., and Nami, S.A.A., Russ. J. Gen. Chem., 2019, vol. 89, pp. 1851–1858. https://doi.org/10.1134/S1070363219090202
Arshad, M., Int. J. Pharm. Sci. Res., 2018, vol. 9, pp. 35–41. https://www.ijpsr.info/docs/IJPSR18-09-02-017.pdf.
Alodeani, E.A., Arshad, M., Izhari, M.A. Europ.J. Biomed. and Pharm. Sci., 2014, vol. 1, pp. 504–527. http://www.ejbps.com/admin/assets/article_issue/ volume_1_december_issue_3/1419598913.pdf.
Arshad, M., Bhat, A.R., Hoi, K.K., Choi, I., and Athar, F, Chin. Chem. Lett., 2017, vol. 28, pp. 1559–1565. https://doi.org/10.1016/j.cclet.2016.12.037
Arshad, M., Khan, M.S., Nami, S.A.A., and Ahmad, D., SNAppl. Sci., 2019, vol. 1, pp. 1–8. https://doi.org/10.1007/s42452-019-0571-8
Iram, N.E., Khan, M.S., Jolly, R., Arshad, M., Alam Alam, P., Khan, R.H., and Firdaus, F., J. Photochem. Photobiol. B: Biol., 2015, vol. 153, pp. 20–32. https://doi.org/10.1016/j.jphotobiol.2015.09.001
Nami, S.A.A., Arshad, M., Shakir, M., Khan, M.S., Alam, M., Lee, D.-U., Park, S., and Sarikavakli, N., Polym. Adv. Technol., 2015, vol. 26, pp. 1627–1638. https://doi.org/10.1002/pat.3591
Nami, S.A.A., Khan, M.S., Arshad, M., Raza M.A., and Khan, I., Polym. Adv. Technol., 2017, vol. 28, pp. 10–19. https://doi.org/10.1002/pat.3846
Kareema, A., Laxmi, Arshad M., Nishat, N., J. Photochem. Photobiol. B: Biol., 2016, vol. 160, pp. 163–171. https://doi.org/10.1016/j.jphotobiol.2016.03.030
Gupta, M.K., Neelakantan, T.V., Sanghamitra, M., Tyagi, R.K., Dinda, A., Maulik, S., Mukhopadhyay, C.K., and Goswami, S.K., Antioxid. Redox Signal., 2006, vol. 8, pp. 1081–1093.
Mosmann, T., J. Immunol. Methods., 1983, vol. 65, p. 55. https://doi.org/10.1016/0022-1759(83)90303-4
Nayab, P.S., Arif, R, and Arshad, M., Heterocycl.Lett., 2015, vol. 5, pp. 223–239. https://www.heteroletters.org/issue25/PDF/Paper-9.pdf.
Arshad, M., SNAppl. Sci., 2020, vol. 2, p. 467. https://doi.org/10.1007/s42452-020-2243-0
ACKNOWLEDGMENTS
The author, Dr. Mohammad Arshad, is highly thankful to the Dean, College of Medicine, Al-Dawadmi, Shaqra University, Kingdom of Saudi Arabia for his cooperation to accomplish this work.
Funding
There was no funding allotted for the study the authors performed the study using their own resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Corresponding author: phone: +966559712511; e-mail: mohdarshad1985@gmail.com; m.arshad@su.edu.sa.
Rights and permissions
About this article
Cite this article
Mohammad Arshad Design, Drug-Likeness, Synthesis, Characterization, Antimicrobial Activity, Molecular Docking, and MTT Assessment of 1,3-Thiazolidin-4-one Bearing Piperonal and Pyrimidine Moieties. Russ J Bioorg Chem 46, 599–611 (2020). https://doi.org/10.1134/S1068162020040056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020040056