Abstract
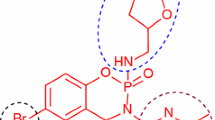
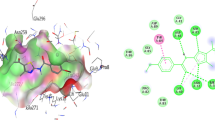
The synthesis of a new series of S-substituted acetamides derivatives of 5-[(2-amino-1,3-thiazol-4-yl)methyl]-1,3,4-oxadiazol-2-thiol were synthesized and evaluated for enzyme inhibition study along with cytotoxic behavior. Ethyl 2-(2-amino-1,3-thiazol-4-yl)acetate was converted to corresponding acid hydrazide by hydrazine hydrate in ethanol. The reflux of acid hydrazide with carbon disulfide resulted to 5-[(2-amino-1,3-thiazol-4-yl)methyl]-1,3,4-oxadiazol-2-thiol. Different electrophiles were synthesized by the reaction of respective anilines (one in each reaction) and 2-bromoacetylbromide in an aqueous medium. The targeted bi-heterocyclic compounds were synthesized by stirring nucleophilic 5-[(2-amino-1,3-thiazol-4-yl)methyl]-1,3,4-oxadiazol-2-thiol with different acetamides electrophiles (one after another), in DMF using LiH as base and activator. The proposed structures of newly synthesized compounds were deduced by spectroscopic techniques such as 1H NMR, 13C NMR, EI MS and elemental analysis. These novel bi-heterocycles were tested for their anti-diabetic potential via the in vitro inhibition of \(\alpha \)-glucosidase enzyme. The in silico study of these molecules was also coherent with their enzyme inhibition data. Furthermore, these molecules were analyzed for their cytotoxic behavior against brine shrimps. It was inferred from the results that most of them exhibited very potent inhibitory potential against the studied enzyme and can be utilized as valuable anti-diabetic agent.

Similar content being viewed by others
REFERENCES
Pasqualotto, A.C., Thiele, K.O., and Goldani, L.Z., Eur. J. Med. Chem., 2010, vol. 11, pp. 165–169.
Knadler, M.P., Bergstrom, R.F., Callaghan, J.T., and Rubin, A., Asian J. Chem., 1986, vol. 14, pp. 175–187.
Foroumadi, A., Mansouri, S., Kiani, Z., and Rahmani, A., Eur. J. Med. Chem., 2003, vol. 38, pp. 851–857.
Yatin, J.M., Arun, M.I., Shridhar, M., Shrikrishna, I., and Hoong-Kun, F., Arab. J. Chem., 2013, vol. 6, pp. 177–184.
Cihan-ustundag, G., Simsek, B., Ilhan, E., and Capan, G., Lett. Drug Design Disc., 2014, vol. 11, pp. 290–296.
Holla, B.S., Veerendra, B., Shivananda, M.K., and Poojary, B., Eur. J. Med. Chem., 2003, vol. 38, pp.759–765.
Abdel-Rahman F., Erik, D.C., and Elkashef, H., Arkivoc, 2006, pp. 137–141.
Li, Z., Zhan, P., and Liu, X., Mini. Rev. Med. Chem., 2011, vol. 11, pp. 1130–1142.
Joshi, D. and Parikh, K.S., Med. Chem. Res., 2014, vol. 23, pp. 1855–1864.
Bondock, S., Adel, S., Etman, H.A., and Badria, F.A., Eur. J. Med. Chem., 2012, vol. 48, pp. 192–199.
Patel, K.N., Jadhav, N.C., Jagadhane, P.B., and Telvekar, V.N., Synlett., 2012, vol. 23, pp. 1970–1972.
Gupta, A., Kashaw, S.K., Jain, N., Rajak, H., Soni, A., Stables, J.P., Med. Chem. Res., 2011, vol. 20, pp. 1638–1642.
Sanchit, S. and Pandeya, S.N., Int. J. Res. Ayurv. Pharm., 2011, vol. 2, pp. 459–468.
Coppo, F.T., Evans, K.A., Graybill, T.L., and Burton, G., Tetrahedron Lett., 2004, vol. 45, pp. 3257–3260.
Yang, S.J., Lee, S.H., Kwak, H.J., and Gong, Y.D., J. Org. Chem., 2013, vol. 78, pp. 438–444.
Brain, C.T., Paul, J.M., Loong, Y., and Oakley, P., Tetrahedron Lett., 1999, vol. 40, pp. 3275–3278.
Park, Y.-D., Kim, J.-J., Chung, H.-A., Kweon, D.-H., Cho, S.-D., Lee, S.-G., and Yoon, Y.J., Synthesis, 2003, vol. 4, pp. 560–564.
Patel, R.V., Kumari, P., and Chikhalia, K.H., Med. Chem., 2013, vol. 9, pp. 596–607.
Oliveira, C.S., Lira, B.F., Barbosa-Filho, J.M., Lorenzo, J.G., and Athayde-Filho, P.F., Molecules, 2012, vol. 17, pp. 10 192–10 231.
Kitchen, D.B., Decornez, H., Furr, J.R., and Bajorath, J., Nat. Rev. Drug Discov., 2004, vol. 3, pp. 935–949.
Sama, K., Murugesan, K., and Sivaraj, R., AsianJ. Plant Sci. Res., 2012, vol. 2, pp. 550–553.
Abbasi, M.A., Ramzan, M.S., Aziz-ur-Rehman, Siddiqui, S.Z., Hassan, M., Raza, H., Shah, S.A.A., Mirza, B., and Seo, S.-Y., J. Serb. Chem. Soc., 2019, vol. 84, pp. 649–661.
Abbasi, M.A., Ramzan, M.S., Aziz-ur-Rehman, Siddiqui, S.Z., Shah, S.A.A., Hassan, M., Seo, S.-Y., Ashraf, M., Mirza, B., and Ismail, H., Russ. J. Bioorg. Chem., 2018, 44, pp. 801–811.
Butt, A.R.S., Abbasi, M.A., Aziz-ur-Rehman, Siddiqui, S.Z., Hassan, M., Raza, H., Shah, S.A.A., and Seo, S.-Y., Bioorg. Chem. 2019, vol. 86, pp. 197–209.
Ellman, G.L., Courtney, K.D., Andres, V., Featherstone, R.M., Bio Pharm., 1961, vol. 7, pp. 88–95.
Tan, K., Tesar, C., Wilton, R., Keigher, L., Babnigg, G., Joachimiak, A., FASEB J., 2010, vol. 24, pp. 3939–3949.
Boström, J., Greenwood, J.R., and Gottfries, J., Mol. Graph. Model., 2003, vol. 21, pp. 449–462.
Jensen, E.C., Ogg, C., Nickerson, K., App. Biochem. Microb., 1992, vol. 58, pp. 2505–2508.
FUNDING
The Higher Education Commission (HEC) of Pakistan is highly acknowledged by the authors for financial support regarding purchasing of chemicals and spectral study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interests.
Additional information
Corresponding author: phone: (+92)-42-111000010; e-mail: atrabbasi@yahoo.com; abbasi@gcu.edu.pk
Rights and permissions
About this article
Cite this article
Muhammad Athar Abbasi, Ramzan, M.S., Aziz-ur-Rehman et al. Synthesis of Novel Bi-Heterocycles as Valuable Anti-Diabetic Agents: 2-({5-((2-Amino-1,3-Thiazol-4-yl)methyl)-1,3,4-Oxadiazol-2-yl}sulfanyl)-N-(Substituted)acetamides. Russ J Bioorg Chem 46, 590–598 (2020). https://doi.org/10.1134/S1068162020040020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020040020