Abstract
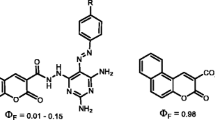
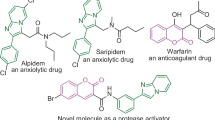
A new series of 5,6-benzocoumarin derivatives carrying anti-inflammatory drugs were synthesized via alkylamide spacers, the target to develop novel imaging fluorescent agents as useful technique to cancer early discovery. Experimentally, treatment of N-Boc-1,3-propanediamine with ethyl 5,6-benzocoumarin-3-carboxlate (III) under reflux conditions followed by hydrolyzed with CF3COOH to the corresponding primary amine (V), then treatment of derivative (V) with anti-inflammatory drugs have carboxylic group in presence of DCC (N,N'-dicyclohexylcarbodiimide) as catalyst and MeCN as solvent. The structure of the synthesized compounds was confirmed by FT-IR, NMR (1H,13C) spectral data as well as elemental analysis. The fluorescence properties study was investigated spectrophotometrically in methanol and maximum emission (λem) exhibited within range 411–436 nm, meanwhile, the quantum yields were calculated comparison to Rhodamine 6G as reference. Interestingly, the compound (III) gave a higher quantum yield (ΦF = 0.96), meanwhile, compounds (VII, VIII, IX and XI) gave reasonable quantum yields (ΦF) 0.60, 0.65, 0.78 and 0.84, respectively. All synthesized compounds were screened for their anti-AChE and antimicrobial activity. Based on the results, some of the compounds showed good activity in compared to the standard drugs.


Similar content being viewed by others
REFERENCES
Kontogiorgis, C.A. and Hadjipavlou-Litina, D.J., Bioorg. Med. Chem. Lett., 2004, vol. 14, pp. 611–614.
Al-Soud, Y.A., Al-Sauodoni, H.H., Amajaour, H.A.S., Salih, K.S.M., Mubarak, M.S., Al-Masoudi, N.A., and Jaber, I.H., Z. Naturforsch., 2008, vol. 63, pp. 83–89.
Emami, S. and Dadashpour, S., Eur. J. Med. Chem., 2015, vol. 102, pp. 611–630.
Lv, H.N., Wang, S., Zeng, K.W., Li, J., Guo, X.Y., Ferreira, D., Zjawiony, J.K., Tu, P.F., and Jiang, Y., J. Nat. Prod., 2015, vol. 78, pp. 279–285.
Zaki, R.M., Elossaily, Y.A., and El-Dean, A.M., Russ. J. Bioorg. Chem., 2012, vol. 38, pp. 639–646.
Salem, M.A.I., Marzouk, M.I., and El-Kazak, A.M., Molecules, 2016, vol. 21, pp. 249–256.
Sashidhara, K.V., Palnati, G.R., Avula, S.R., Singh, S., Jain, M., and Dikshit, M., Bioorg. Med. Chem. Lett., 2012, vol. 22, pp. 3115–3121.
Al-Soud, Y.A., Al-Sa’doni, H.H., Amajaour, H.A.S., Salih, K.S.M., Mubarakb, M.S., and Al-Masoudic, N.A., Z. Naturforsch., 2008, vol. 63, pp. 83–89.
Reddy D.S., Hosamani K.M., Devarajegowda H.C., Eur. J. Med. Chem., 2015, vol. 101, pp. 705–715.
Sashidhara, K.V., Kumar, M., Modukuri, R.K., Srivastava, A., and Puri, A., Bioorg. Med. Chem. Lett., 2011, vol. 21, pp. 6709–6713.
Abdel-Aziem, A., Rashdan, H.R.M., Ahmed, E.M., and Shabaan, S.N., Green Chem. Lett. Rev., 2019, vol. 12, pp. 9–18.
Webb, D.J. and Brown, C.M., Methods Mol. Biol., 2012, vol. 931, pp. 29–59.
Al-Masoudi, N.A., Al-Salihi, N.J., Marich, Y.A., and Markus, T., J. Fluoresc., 2015, vol. 25, pp. 1847–1854.
Zaizafoon N., Int. J. Biochem. Res. Rev., 2015, vol. 7, pp. 100–111.
Rajesha, G., Kiran Kumar, H.C., Bhojya Naik, H.S., and Mahadevan, K.M., S. Afr. J. Chem., 2011, vol. 64, pp. 88–94.
Egorove, N.S., Antibiotics Scientific Approach, Moscow: Mir, 1985.
ACKNOWLEDGMENTS
All authors are extremely thankful to Department of Chemistry, College of Education, University of Al- Qadisiyah for providing necessary facilities. We also thank Dr. Najim A. Al-Masoudi for his sustain guidance to carry out the lab work. Dr. Zaizafoon Nabeel for do the biological evaluation experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Corresponding author: phone: +9647809679779; e-mail: qassimjaber99@gmail.com.
Supplementary material
Rights and permissions
About this article
Cite this article
Nabeel A. Abdul-Rida, Adnan, S. & Jaber, Q.A. Development of Novel Imaging Fluorescent Agents Bearing Anti-Inflammatory Drugs: Synthesis, Structural Characterization and Evaluation of Biological Activity. Russ J Bioorg Chem 46, 620–626 (2020). https://doi.org/10.1134/S1068162020040032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020040032