Abstract
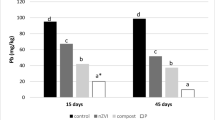
Nano zerovalent iron (nZVI) is one of the most widely used nanotechnologies in soil remediation, but its long-term performance can be limited due to its high reactivity and rapid ageing. The aim of this study was to compare the immobilisation of Cd, Cu, Ni, and Pb in artificially contaminated soil samples using nZVI and to evaluate their retention over a 1-year period. Single-metal-contaminated soil samples were amended using 0–1.05% of nZVI. Leaching, using CaCl2 solution and a sequential extraction procedure, was performed to evaluate the immobilisation efficiency and fractionation of heavy metals before and after ageing. The results of the extractions showed that the application of nZVI initially reduced the exchangeable fraction of all heavy metals. The greatest immobilisation efficiency was achieved for Cu. The retention of heavy metals by nZVI was studied by constantly humidifying soil samples for a 12-month period. The results showed that over this period, the efficiency of nZVI treatment decreased, due to the decrease in the amount of reactive iron oxides in soil samples. Retention of immobilised heavy metals by nZVI strongly depended on their ionic radius. The highest retention results were obtained for Cu and Ni, whereas the lowest results were obtained for Cd and Pb.


Similar content being viewed by others
References
Abdel-Samad, H., & Watson, P. R. (1998). An XPS study of the adsorption of lead on goethite (α-FeOOH). Applied Surface Science, 136(1–2), 46–54.
Alvarez, M., Horst, M. F., Sileo, E. E., & Rueda, E. H. (2012). Effect of Cd (II) on the ripening of ferrihydrite in alkaline media. Clays and Clay Minerals, 60(2), 99–107.
Bouyoucos, G. J. (1962). Hydrometer method improved for making particle size analyses of soils 1. Agronomy Journal, 54(5), 464–465.
Calderon, B., & Fullana, A. (2015). Heavy metal release due to aging effect during zero valent iron nanoparticles remediation. Water Research, 83, 1–9.
Cismasu, A. C., Michel, F. M., Tcaciuc, A. P., Tyliszczak, T., & Brown Jr., G. E. (2011). Composition and structural aspects of naturally occurring ferrihydrite. Comptes Rendus Geoscience, 343(2–3), 210–218.
Danila, V., Vasarevicius, S., & Valskys, V. (2018). Batch removal of Cd (II), Cu (II), Ni (II), and Pb (II) ions using stabilized zero-valent iron nanoparticles. Energy Procedia, 147, 214–219.
Danila, V., Kumpiene, J., Kasiuliene, A., & Vasarevičius, S. (2020). Immobilisation of metal (loid) s in two contaminated soils using micro and nano zerovalent iron particles: Evaluating the long-term stability. Chemosphere, 248, 126054.
Degryse, F., Smolders, E., & Parker, D. R. (2009). Partitioning of metals (Cd, Co, Cu, Ni, Pb, Zn) in soils: Concepts, methodologies, prediction and applications–a review. European Journal of Soil Science, 60(4), 590–612.
Dold, B. (2003). Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. Journal of Geochemical Exploration, 80(1), 55–68.
Efecan, N., Shahwan, T., Eroğlu, A. E., & Lieberwirth, I. (2009). Characterization of the uptake of aqueous Ni2+ ions on nanoparticles of zero-valent iron (nZVI). Desalination, 249(3), 1048–1054.
Emadi, M., Savasari, M., Bahmanyar, M. A., & Biparva, P. (2019). Application of stabilized zero valent iron nanoparticles for immobilization of lead in three contrasting spiked soils. Research on Chemical Intermediates, 45(9), 4261–4274.
Fajardo, C., Sánchez-Fortún, S., Costa, G., Nande, M., Botías, P., García-Cantalejo, J., et al. (2020). Evaluation of nanoremediation strategy in a Pb, Zn and Cd contaminated soil. Science of the Total Environment, 706, 136041.
Gil-Díaz, M., Pinilla, P., Alonso, J., & Lobo, M. C. (2017). Viability of a nanoremediation process in single or multi-metal (loid) contaminated soils. Journal of Hazardous Materials, 321, 812–819.
Gong, Y., Liu, Y., Xiong, Z., Kaback, D., & Zhao, D. (2012). Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology, 23(29), 294007.
Hendershot, W. H., & Duquette, M. (1986). A simple barium chloride method for determining cation exchange capacity and exchangeable cations 1. Soil Science Society of America Journal, 50(3), 605–608.
Houba, V. J. G., Temminghoff, E. J. M., Gaikhorst, G. A., & Van Vark, W. (2000). Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Communications in Soil Science and Plant Analysis, 31(9–10), 1299–1396.
Huang, B., Li, Z., Huang, J., Chen, G., Nie, X., Ma, W., et al. (2015). Aging effect on the leaching behavior of heavy metals (Cu, Zn, and Cd) in red paddy soil. Environmental Science and Pollution Research, 22(15), 11467–11477.
ISO 14238. (2012). Soil quality — Biological methods — Determination of nitrogen mineralization and nitrification in soils and the influence of chemicals on these processes. Geneva:International Organization for Standardization.
Jalali, M., & Khanlari, Z. V. (2008). Effect of aging process on the fractionation of heavy metals in some calcareous soils of Iran. Geoderma, 143(1–2), 26–40.
Jiang, D., Zeng, G., Huang, D., Chen, M., Zhang, C., Huang, C., & Wan, J. (2018). Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environmental Research, 163, 217–227.
Jiang, K., Zhou, K., & Zhang, J. (2019). Rapid determination of the immobilization conditions for lead and cadmium in soil using 2, 4, 6-trimercaptotriazine, trisodium salt, nonahydrate. Journal of Environmental Engineering and Landscape Management, 27(4), 209–214.
Karabelli, D., ÜzÜm, C., Shahwan, T., Eroglu, A. E., Scott, T. B., Hallam, K. R., & Lieberwirth, I. (2008). Batch removal of aqueous Cu2+ ions using nanoparticles of zero-valent iron: A study of the capacity and mechanism of uptake. Industrial & Engineering Chemistry Research, 47(14), 4758–4764.
Kim, H. S., Kim, T., Ahn, J. Y., Hwang, K. Y., Park, J. Y., Lim, T. T., & Hwang, I. (2012). Aging characteristics and reactivity of two types of nanoscale zero-valent iron particles (FeBH and FeH2) in nitrate reduction. Chemical Engineering Journal, 197, 16–23.
Kim, R. Y., Yoon, J. K., Kim, T. S., Yang, J. E., Owens, G., & Kim, K. R. (2015). Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environmental Geochemistry and Health, 37(6), 1041–1061.
Kinniburgh, D. G., Jackson, M. L., & Syers, J. K. (1976). Adsorption of alkaline earth, transition, and heavy metal cations by hydrous oxide gels of iron andaluminum. Soil Science Society of America Journal, 40(5), 796–799.
Komárek, M., Vaněk, A., & Ettler, V. (2013). Chemical stabilization of metals and arsenic in contaminated soils using oxides–a review. Environmental Pollution, 172, 9–22.
Kumpiene, J., Lagerkvist, A., & Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Management, 28(1), 215–225.
Kumpiene, J., Fitts, J. P., & Mench, M. (2012). Arsenic fractionation in mine spoils 10 years after aided phytostabilization. Environmental Pollution, 166, 82–88.
Kumpiene, J., Antelo, J., Brännvall, E., Carabante, I., Ek, K., Komárek, M., et al. (2019). In situ chemical stabilization of trace element-contaminated soil–field demonstrations and barriers to transition from laboratory to the field–A review. Applied Geochemistry, 100, 335–351.
Li, X. Q., & Zhang, W. X. (2007). Sequestration of metal cations with zerovalent iron nanoparticles a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). The Journal of Physical Chemistry C, 111(19), 6939–6946.
Li, S., Wang, W., Liang, F., & Zhang, W. X. (2017). Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. Journal of Hazardous Materials, 322, 163–171.
Li, S., Chen, S., Wang, M., Lei, X., Zheng, H., Sun, X., et al. (2020). Iron fractions responsible for the variation of Cd bioavailability in paddy soil under variable pe+ pH conditions. Chemosphere, 126355.
Liu, R., & Zhao, D. (2007). In situ immobilization of Cu (II) in soils using a new class of iron phosphate nanoparticles. Chemosphere, 68(10), 1867–1876.
Liu, A., Liu, J., & Zhang, W. X. (2015). Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water. Chemosphere, 119, 1068–1074.
Liu, B., Ma, X., Ai, S., Zhu, S., Zhang, W., & Zhang, Y. (2016). Spatial distribution and source identification of heavy metals in soils under different land uses in a sewage irrigation region, Northwest China. Journal of Soils and Sediments, 16(5), 1547–1556.
Lock, K., & Janssen, C. R. (2003). Influence of ageing on zinc bioavailability in soils. Environmental Pollution, 126(3), 371–374.
Ma, Y., Lombi, E., Oliver, I. W., Nolan, A. L., & McLaughlin, M. J. (2006). Long-term aging of copper added to soils. Environmental Science & Technology, 40(20), 6310–6317.
Makaras, T., Montvydienė, D., Kazlauskienė, N., & Stankevičiūtė, M. (2019). Rapidness-and sensitivity-based comparison of behavioral and respiratory responses of European perch and rainbow trout to metal mixture exposure. Bulletin of Environmental Contamination and Toxicology, 103(3), 391–399.
Meyer, W. L., & Arp, P. A. (1994). Exchangeable cations and cation exchange capacity of forest soil samples: Effects of drying, storage, and horizon. Canadian Journal of Soil Science, 74(4), 421–429.
Mohamadiun, M., Dahrazma, B., Saghravani, S. F., & Darban, A. K. (2018). Removal of cadmium from contaminated soil using iron (III) oxide nanoparticles stabilized with polyacrylic acid. Journal of Environmental Engineering and Landscape Management, 26(2), 98–106.
Pan, B., & Xing, B. (2012). Applications and implications of manufactured nanoparticles in soils: A review. European Journal of Soil Science, 63(4), 437–456.
Pullin, H., Springell, R., Parry, S., & Scott, T. (2017). The effect of aqueous corrosion on the structure and reactivity of zero-valent iron nanoparticles. Chemical Engineering Journal, 308, 568–577.
Sun, Y. P., Li, X. Q., Cao, J., Zhang, W. X., & Wang, H. P. (2006). Characterization of zero-valent iron nanoparticles. Advances in Colloid and Interface Science, 120(1–3), 47–56.
Tiberg, C., Kumpiene, J., Gustafsson, J. P., Marsz, A., Persson, I., Mench, M., & Kleja, D. B. (2016). Immobilization of Cu and As in two contaminated soils with zero-valent iron–long-term performance and mechanisms. Applied Geochemistry, 67, 144–152.
Vasarevičius, S., Danila, V., & Paliulis, D. (2019). Application of stabilized nano zero valent iron particles for immobilization of available Cd 2+, Cu 2+, Ni 2+, and Pb 2+ ions in soil. International Journal of Environmental Research, 13(3), 465–474.
Wang, Q., Lee, S., & Choi, H. (2010). Aging study on the structure of Fe0-nanoparticles: Stabilization, characterization, and reactivity. The Journal of Physical Chemistry C, 114(5), 2027–2033.
Wu, S., Cajthaml, T., Semerád, J., Filipová, A., Klementová, M., Skála, R., et al. (2019). Nano zero-valent iron aging interacts with the soil microbial community: A microcosm study. Environmental Science: Nano, 6(4), 1189–1206.
Xu, Y., & Zhao, D. (2007). Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Research, 41(10), 2101–2108.
Zhang, M., Wang, Y., Zhao, D., & Pan, G. (2010). Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe 3 O 4) particles. Chinese Science Bulletin, 55(4–5), 365–372.
Zhang, X., Yang, L., Li, Y., Li, H., Wang, W., & Ye, B. (2012). Impacts of lead/zinc mining and smelting on the environment and human health in China. Environmental Monitoring and Assessment, 184(4), 2261–2273.
Zhang, Y., Su, Y., Zhou, X., Dai, C., & Keller, A. A. (2013). A new insight on the core–shell structure of zerovalent iron nanoparticles and its application for Pb (II) sequestration. Journal of Hazardous Materials, 263, 685–693.
Zhao, X., Liu, W., Cai, Z., Han, B., Qian, T., & Zhao, D. (2016). An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Research, 100, 245–266.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vasarevičius, S., Danila, V. & Januševičius, T. Immobilisation of Cadmium, Copper, Lead, and Nickel in Soil Using Nano Zerovalent Iron Particles: Ageing Effect on Heavy Metal Retention. Water Air Soil Pollut 231, 496 (2020). https://doi.org/10.1007/s11270-020-04864-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04864-9