Abstract
We present a list of Ostracoda (Crustacea) from stratigraphic sections of Mio–Pleistocene lacustrine deposits from Zhada Basin, western Tibetan Plateau. In this area, almost no taxonomical studies were carried out so far, and, aiming to a future use of ostracods as palaeoenvironmental proxy for this sector of the Tibetan Plateau, a documentation of several species was performed. The taxa Leucocytherella sinensis Huang, 1982, ?Leucocythere dorsotuberosa Huang, 1982, Leucocythere postilirata Pang, 1985, Ilyocypris spp., Eucypris cf. zandaensis Yang, 1982, ?Prionocypris sp., Paraeucypris sp. and Leucocytherella dangeloi sp. nov. were found and classified. The taxon Ilyocypris spp. probably represents three different species; other taxa in open nomenclature are Paraeucypris sp., Eucypris cf. zandaensis and ?Prionocypris sp. The reported taxa from the Zhada Basin are mainly lacustrine species, and their diversity is comparable to those of other Neogene and Quaternary basins located on the Tibetan Plateau.
Similar content being viewed by others
Introduction
There are many examples for the prominent role of Ostracoda (Crustacea) in different fields of geosciences and their use as palaeoenvironmental, palaeoclimatic and biostratigraphic indicators (Boomer et al. 2003; Horne 2003, 2007). Their sensitivity to environmental changes and their wide distribution in all types of water bodies call for their good documentation also in less studied areas. In contrast to the large number of geological and palaeontological studies on the Tibetan Plateau, research on ostracods in this area is rather rare and improved only in the last decade (Wrozyna et al. 2009; Frenzel et al. 2010; Mischke 2012). Investigations were mainly conducted in the more easily accessible northern and eastern part of the plateau (Zhang et al. 1989, 1994, 2006; Mischke et al. 2010), and only a few studies improved the available knowledge of the local ostracod fauna of its central and western part (Li et al. 1991; Zhu et al. 2010; Guo et al. 2016; Song et al. 2017; Alivernini et al. 2018a, b). Furthermore, ostracod studies in the central and southern parts of the Tibetan Plateau focussed on Holocene and Late Pleistocene faunas whereas the precursors of these mainly endemic species are not known. Investigations on Mio–Pleistocene ostracods from the Tibetan Plateau are restricted to its northeastern part so far (Sun 1988; Yang et al. 1997; Mischke et al. 2006, 2010; Wu et al. 2011; Lu et al. 2019) where they are a valuable tool for biostratigraphy in hydrocarbon exploration.
This work focuses on Mio–Pleistocene ostracods of the Zhada Basin located in the western Tibetan Plateau (Fig. 1). Previous works carried out in this area concern its tectonic origins (Wang et al. 2004, 2008; Saylor et al. 2010a) and environmental history (Saylor et al. 2010b) using mostly sedimentological and pollen analyses. Kempf et al. (2009), who investigated petrographic and sedimentological properties, were the first to describe also elements of the ostracod fauna in this area. They found some typical endemic taxa like Leucocytherella sinensis and several not identified species. In this work, we present the Mio–Pleistocene ostracod assemblage recovered from 105 samples of Joel Saylor’s stratigraphic “South Zhada” (“SZ”) section (Fig. 2), located in the southern part of the Zhada Basin and already sedimentologically analysed and dated by Saylor (2008) to improve the taxonomic database on ostracods of this area for future palaeoecological and potentially stratigraphical studies.
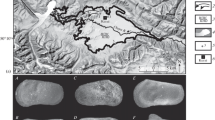
Location of the Zhada Basin on the Tibetan Plateau (a) and focus of the sampling area (b) (Geomapapp image modified). The correspondence between sectors and sample list is explained in Table 1
Lithology and age data for the section SZ (South Zhada) from Saylor et al. 2010a (modified). C claystone, S siltstone, SS sandstone, CgM conglomerate
Study area
The Zhada Basin is the largest late Cenozoic sedimentary basin in the Tibet Autonomous Region. It is located north of the high Himalayan ridge crest in the western part of the orogen (~ 32° N, 82° E; Fig. 1). The basin is at least 150 km long and 60 km wide, and the current outcrop extent of the basin fill covers at least 9000 km2 (Saylor et al. 2010a). It is bounded by the South Tibetan detachment system to the southwest, the Indus suture to the northeast, and the Leo Pargil and Gurla Mandhata gneiss domes to the northwest and southeast, respectively (Saylor et al. 2010b). The Zhada Basin contains a thick sequence of late Neogene and early Quaternary fluvial and lacustrine deposits (Kempf et al. 2009) which allows the reconstruction of long-term climate history.
Materials and methods
Fieldwork
A total of 124 sediment samples from seven, relatively thick lake beds were collected from Saylor’s “South Zhada” section (Saylor 2008) in 2012 (Table 1). The selected lake beds are distributed more or less evenly over the 820-m-thick sediment sequence to enable the investigation and comparison of ostracods from stagnant-water deposits formed over the last ca. 8 million years as estimated by Saylor et al. (2010a). Sediment samples from individual lake beds were collected at ca. 0.5-m intervals (Table 1). The seven selected lake beds are located between 31.46538° N and 79.72865° E as the lowermost and northernmost position and 31.36556° N and 79.75152° E as the uppermost and southernmost position.
Micropalaeontological analysis
All 124 sediment samples were prepared for micropalaeontological analysis. The samples were treated with H2O2 (ca. 5–10% for about 1–2 h) to separate aggregates of mud, and they were subsequently sieved with water through a 200-µm-sieve to remove fine-grained particles. For quantitative ostracod analysis, the sieve residues were split into sub-samples using a microsplitter. Sixteen samples were barren of ostracods. The species proportions and the relative ostracod abundances were calculated considering all ontogenetic stages (juvenile and adult valves). To assess water turbulence (Boomer et al. 2003) and the possible removal of thinner and smaller juvenile valves by dissolution, the adult/juvenile ratio was determined. Identification was performed primarily with a low-power binocular microscope and was occasionally supported by a scanning electron microscope (SEM) as well as a Keyence Digital Microscope. The valves were classified and taxonomically attributed, where possible, by comparison with previous studies of ostracods from the Tibetan Plateau (Wrozyna et al. 2009, 2010; Mischke et al. 2010; Akita et al. 2016) and using Chinese literature (Huang 1982; Hou et al. 2002; Hou and Gou 2007). In addition, an amended description of the valves was added.
Results
Presence of organism remains and preservation
In total, 105 sediment samples contained ostracod valves. From these samples, 6722 ostracod valves were recovered. Most valves are disarticulated, and adult valves are dominant. Juvenile valves, especially from silty to fine sandy sediments, are often deformed. Besides ostracod valves, gyrogonites of charophytes and mollusc shells, mostly fragments of Gastropoda, were found frequently in the samples.
Ostracod taxonomy
We recorded at least eight ostracod species in the 124 samples from the Zhada Basin (Table 2). The most abundant species is L. sinensis (Huang 1982) which often occurs in association with ?Leucocythere dorsotuberosa and L. postilirata (Table 2). Other abundant taxa are Paraeucypris sp. and Leucocytherella dangeloi sp. nov.
A systematic overview of the ostracod taxa of the Zhada Basin follows below. The synonymy lists contain selected papers as first description, emendations and other taxonomically important references. The systematic description is adopted from Martin and Davis (2001) and complemented from Fürstenberg et al. (2015).
Class Ostracoda Latreille, 1802
Order Podocopida Müller, 1894
Suborder Cytherocopina Baird, 1850
Superfamily Cytheroidea Baird, 1850
Family Limnocytheridae Klie, 1938
Subfamily Limnocytherinae Klie, 1938
Genus Leucocytherella Huang, 1982
Leucocytherella sinensis Huang, 1982
Figure 3a, b
Leucocytherella sinensis a left valve external view, sample Z063; b right valve internal view, sample Z063. ?Leucocythere postilirata c LV ext., sample Z0104; d RV int., sample Z037; e RV int., sample Z037. ?Leucocythere dorsotuberosa f RV ext., sample Z0110; g RV ext., sample Z0112; h RV int. juv., sample Z0112
- 1982:
-
Leucocytherella sinensis Huang gen. et sp. nov.—Huang: 341–342; text-figs. 23–26; pl. 12: figs. 1–8; pl. 13: figs. 1–7 [type species of Leucocytherella Huang, 1982]
- 2015:
-
Leucocytherella sinensis Huang—Fürstenberg et al.: 67–70; figs. 6, 10–12 [comprehensive synonymy list]
- 2016:
-
Leucocytherella sinensis Huang—Akita et al.: 7; figs. 3/6–10
- 2016:
-
Leucocytherella sinensis Huang—Guo et al.: fig. 2 [upper left valve]
- 2018a:
-
Leucocytherella sinensis Huang—Alivernini et al.: fig. 8/1
Studied material. 3202 valves (adults and juveniles, females and males, including specimens with complete carapaces).
Size. 0.64–0.75 mm (male); 0.64–0.72 mm (female).
Original description. (Huang 1982). Valve of female rectangular in lateral view, anterior end higher than posterior, two transverse sulci anterodorsally, radial pore canal zone moderately broad, with slender, straight and sparse radial pore canals. Hinge of left valve consists of an anterior small reniform tooth, a posterior small triangular one and middle shallow groove. Valve of male rather long, both ends nearly equivalently high. Valve of juveniles rather short, anterior end higher than posterior.
For a detailed description of the valves of recent specimens (adult and juveniles, males, females,) on the Tibetan Plateau see Fürstenberg et al. (2015).
Stratigraphic, ecological and geographic distribution. From Miocene to recent (Huang 1987). Leucocytherella sinensis is ubiquitous and endemic on the Tibetan Plateau above 4000 m above sea level (Akita et al. 2016). Valves of L. sinensis were found in lakes, ponds, rivers, and lagoon-like and estuary-like water bodies at lake shores in salinities of 0.08–12.81 psu. Specimens live on mud, sand, sandy gravel and in phytal habitats in permanent fresh to brackish lacustrine waters, preferentially in Ca2+ depleted waters. The nodes on the calcitic valves are more numerous and pronounced at low salinity and can be used as a proxy for palaeosalinity (Fürstenberg et al. 2015).
Leucocytherella dangeloi Alivernini sp. nov.
Figure 4a–g
Etymology. The name “dangeloi” was given to commemorate the death of Fabio D'Angelo, a young micropalaeontologist at the beginning of his academic career who died in 2012.
Holotype. A male right valve (0.70 mm). Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. Collection number: 171681.
ZooBank LSID. The nomenclatural act established herein is registered under urn:lsid:zoobank.org:act:463C6374-8ACB-4AD0-B365-6092BA87EADC.
Studied material. 506 valves (adults and juveniles, females and males, including specimens with complete carapaces).
Size. 0.58–0.72 mm (males); 0.58–0.70 mm (females).
Diagnosis. Typical Leucocytherella species but with smooth surface and posterior part relatively higher compared to anterior part than in L. sinensis, more evident in the left valve. Lophodont hinge.
Locality and age. Zhada Basin, sample Z068 (ca. 4 million years).
Description and comparison. Carapace nearly rectangular. The posterior part of valves of L. dangeloi sp. nov., as well as of L. sinensis, is more rounded and higher than the anterior one, but this difference is more pronounced in L. dangeloi sp. nov. with an even higher rounding (Fig. 5; Table 3). Valves smooth and less pitted than those of L. sinensis. Weak dorsomedial sulcus at half-length of carapace. Protuberance in the anterodorsal part of the carapace. Valves are un-noded or weakly noded (Table 3). Four adductor muscular scars arranged in an almost vertical and slightly inclined row, located slightly anteriorly from the centre of the valve. Marginal pore canals thin, from weakly curved to straight and not numerous. Similar to L. sinensis, the hinge of the right valve consists of a pit on both ends and a ridge in between; the hinge of the left valve presents an anterior small rounded tooth, a small triangular posterior one and a shallow groove in between. Inner posterior lamella is broad. Dorsally, the carapace is sexually dimorphic, males are larger and more expanded posteriorly than females.
Comparison between L. sinensis (a LV ext.; b RV ext.) from Fürstenberg et al. (2015; modified) and L. dangeloi Alivernini sp. nov. (c LV ext; d RV ext). In L. dangeloi the posterior part shows more pronounced rounding than in L. sinensis as well as valves more smooth and less pitted
Stratigraphic distribution. Miocene (Messinian) and Pliocene.
Genus Leucocythere Kaufmann, 1892
?Leucocythere dorsotuberosa Huang, 1982
Figure 3f–h
- 1982:
-
Leucocythere dorsotuberosa Huang—Huang et al.: 335–336; pl. 10: figs. 10–17
- pars 2009:
-
?Leucocythere dorsotuberosa Huang—Wrozyna et al.: 668–669: pl. 2: figs. 2, 4–9, 11 [non 670–671; pl. 2: figs. 1, 3, 10, 12–13 = ?Leucocythere postilirata which is considered as forma of ?L. dorsotuberosa by Wrozyna et al. (2009) who provide a comprehensive synonymy list]
- 2010:
-
Leucocythere dorsotuberosa f. parasculpta Pang, 1985—Zhu et al.: fig. 3/5
- 2010:
-
Leucocythere dorsotuberosa f. typica Huang—Zhu et al.: fig. 3/7
- pars 2010:
-
?Leucocythere dorsotuberosa Huang—Wrozyna et al.: fig. 3/1–3 [non fig. 3/4 = ?L. postilirata]
- non 2011:
-
Leucocythere dorsotuberosa Huang—Wu et al.: 64; pl. 3: fig. 10 [= juvenile Cyprideis torosa]
- 2012:
-
Leucocythere dorsotuberosa Huang—Mischke: fig. 15.3/16–17
- 2016:
-
?Leucocythere dorsotuberosa Huang—Guo et al.: fig. 2 [middle row right]
- 2016:
-
Leucocythere? dorsotuberosa Huang—Akita et al.: 32–33; figs. 3/1–5
- non 2017:
-
Leucocythere dorsotuberosa—Song et al.: fig. 5/7
- 2018a:
-
Leucocythere? dorsotuberosa Huang—Alivernini et al.: fig. 8e–g + 8i
- 2018b:
-
Leucocythere? dorsotuberosa Huang—Alivernini et al.: fig. 3 [3rd from left]
Studied material. 1061 valves (females, males, juveniles, including specimens with complete carapaces).
Size. 0.65–0.77 mm (males); 0.65–0.74 (females).
Original description (Huang 1982: 335). Male valve rectangular, anterior end higher than posterior, dorsal margin nearly straight, ventral margin distinctly concave in the middle. Valves with reticulation. Two transverse sulci anterodorsally, and an alar protuberance extending posteroventrally to medioventrally, and a tubercle in posterodorsal position. Marginal pore-canal zone broad, comprising 10–11% of the length of carapace, marginal pore-canals slender, not numerous, several are furcated, anteriorly with 19 marginal pore-canals. Hinge of the left valve consists of sockets in both sides and a shallow ridge in between; hinge of the right valve consists of elongated teeth at both ends and a groove in between.
Valve of male is longer than that of female, posterior bulgy. Juvenile valve short, anterior broadly rounded, dorsal margin slightly rounded. Hinge of male, female and juvenile are similar. Valves are transparent. Carapaces sub-rectangular in lateral view.
Further description. Wrozyna et al. (2009) observed on recent valves that female carapaces are more triangular, the posterior to anteriomedian region bears protuberances interrupted by a mediodorsal sulcus partly divided by a central node. In dorsal view, anterior and posterior ends are pointed. Right valve overlaps left valve anteroventrally and posteriorly in a lobe-like protrusion (modified from Wrozyna et al. 2009).
Remarks. The found valves of ?L. dorsotuberosa have a lophodont hinge. Wrozyna et al. (2009) and Danielopol et al. (1989) doubted that ?L. dorsotuberosa belongs to the genus Leucocythere because of the different hinge, lophodont in ?L. dorsotuberosa instead of the typically anterior significantly smaller tooth than the posterior one and a crenulated hinge bar of the genus Leucocythere.
Stratigraphic, ecological and geographic distribution. Pliocene to recent (Huang 1982). Living ?L. dorsotuberosa occur mainly in brackish lakes (phytal and muddy substrate) and its marginal lagoon-like water bodies on the Tibetan Plateau. Living individuals have also been found in freshwater, but in low numbers only. Empty valves of ?L. dorsotuberosa were found in higher proportions at larger water depth of modern Tibetan lakes (Akita et al. 2016).
?Leucocythere postilirata Pang, 1985
Figure 3c–e
- 1985:
-
Leucocythere postilirata sp. nov. —Pang: 257; pl. 2: figs. 13–16
- 2009:
-
?Leucocythere dorsotuberosa f. postilirata Pang—Wrozyna et al.: 670–671; pl. 2: figs. 1, 3, 10, 12–13
- 2010:
-
?Leucocythere dorsotuberosa f. postilirata Pang—Wrozyna et al.: fig. 3/4
- 2016:
-
?Leucocythere dorsotuberosa f. postilirata Pang—Akita et al.: fig. 2
- 2018:
-
?Leucocythere dorsotuberosa f. postilirata, Pang—Alivernini et al.: fig. 8/e–f
Studied material. 457 valves (adults and juveniles, females and males, including specimens with complete carapaces).
Size. 0.78–0.92 mm (males); 0.75–0.88 (females).
Original description (Pang 1985: 257). Elongated carapace. Valve of male of elongated kidney shape. Anterior slightly higher and/or has the same height as posterior. Both ends curved. Dorsal margin is elongated and almost straight, mediodorsal slightly curved. The anteromediodorsal area is compressed. Two transverse sulci anterodorsally, the more anterior sulcus shorter than the more posterior one. A rounded node is located between the sulci, another more pronounced bulge behind the posterior sulcus and a third at the end of both sulci. A distinctive anterodorsal carina occurs where the dorsal margin meets the anterior one, another carina runs along the central ventral side below the sulci. A third carina lays posteriorly and protrudes the valve outline. The ventral and posterior carinae are not connected to each other. The maximum width lies at ¼ length of the valve. Valve not curved so much, ornamented with a net of large alveoli. Valves of female shorter than male, kidney shaped. Anterior higher than posterior. Posterior carina and ventral carina weak (modified from Wrozyna et al. 2009).
Further description. As already observed by Wrozyna et al. (2009), the valves present a typical sharp carina running parallel to the ventral margin, more distinct on the right valve. Another more or less developed carina runs parallel to the anteroventral margin. Additionally, a marginal parallel posterior carina following the curvature of the margin can be more or less developed, separated from or fused with the ventral carina. The valves are strongly reticulated.
Remarks. Wrozyna et al. (2009) and Frenzel et al. (2010) regard ?L. postilirata as morphotype of ?L. dorsotuberosa with most pronounced medio-ventral and anterior and often posterior carinae as protruding foldings of the valve. On the basis of the remarkable differences in morphology of both forms, they are discriminated as two species in this paper, as it was done in the original description.
Stratigraphic, ecological and geographic distribution. Living specimens reported from Nam Co and Pumoyong Co; Early Holocene of Peiku Co (Peng 1997), Pleistocene of Kunlun Mountains (Pang 1985), Late Pleistocene of Bangong Lake (Li et al. 1991), Cenozoic of Siling and Bangkok lakes (Pang 1985), Cenozoic of the Qaidam Basin (Sun 1988). Living ?L. postilirata occur where ?L. dorsotuberosa is present. According to Wrozyna et al. (2009), ?L. postilirata shows a higher salinity tolerance (max. 8–10 psu) than ?L. dorsotuberosa in Nam Co, and occurs below the thermocline (20–30 m). Relative abundances increase with water depth.
Suborder Cypridocopina Jones, 1901
Superfamily Cypridoidea Baird, 1845
Family Ilyocyprididae Kaufmann, 1900
Genus Ilyocypris Brady and Norman, 1889
Ilyocypris spp.
Figure 6a–h
Studied material. 676 valves (adults and juveniles, including specimens with complete carapaces).
Remarks. The species of the genus Ilyocypris are often hard to discriminate relying on hard parts only, even in well-studied regions as Central Europe (Meisch 2000). Hou et al. (2002) list eleven Ilyocypris species for the Tibetan Plateau but many of them are of dubious taxonomic state. The partly poor preservation of our material and impossible attribution of most juvenile valves to adult stages makes it difficult to discriminate and identify Ilyocypris species from the Zhada Basin.
All documented valves bear the typical characters of the genus—a rectangular carapace in lateral view, about 1 mm long, with pitted to smooth surface and two conspicuous transverse dorsolateral sulci; the left valve overlaps the right one.
Based on outline and ornamentation, three morphotypes, probably different species, are recognisable: (a) well-rounded anterior and posterior end in lateral view, surface weakly or not pitted, no tubercles; (b) well-rounded anterior and posterior end in lateral view, surface weakly pitted, five distinct tubercles similar to Qinghaicypris subpentanoda Yang, 1982; (c) lateral view with truncated posterior end similar to Ilyocypris inermis Kaufmann, 1900, surface pitted, no tubercles. Left valves of the two well-rounded morphotypes a and b show distinct marginal ripplets on the inner lamella of both ends. This character resembles Ilyocypris bradyi Sars, 1890 and Ilyocypris decipiens Masi, 1905 (Mazzini et al. 2014) but the ripplets are more numerous and can be found at the anterior end as well.
Family Cyprididae Baird, 1845
Subfamily Eucypridinae Baird, 1845
Genus Eucypris Vávra, 1891
Eucypris cf. zandaensis Yang, 1982
Figure 7a, b
- 1982:
-
Eucypris zandaensis Yang sp. nov.—Yang in Huang et al.: 330; pl. 2: figs. 1–9
- 2002:
-
Eucypris zandaensis Yang, 1982—Hou et al.: 169; pl. 19: figs. 5–10
Studied material. 13 valves (adults and juveniles).
Size. 0.78–1.1 mm.
Original description. (Hou et al. 2002) Valves big, female elliptical in lateral view, dorsal margin straight and short inclining to the posterior part in lateral view, ventrally slightly concave, highest point at 2/5 of length, network of lines on the valve, marginal pore channels thick and numerous, central muscle scars with four in front of two others, oviduct traces, male valve longer, traces of four loops of testes recognisable.
Remarks. Our material differs from the male adult description in having a slightly more trapezoidal outline of the right valve and being slightly smaller. Unfortunately, only three adult valves were available.
Distribution. Plio–Pleistocene of Zanda, Zhada Basin (Hou et al. 2002).
Genus Prionocypris Brady and Norman, 1896
?Prionocypris sp.
Figure 7g, h
Studied material. 5 valves (only juveniles).
Size. 0.78–1.00 mm.
Description. Valves rounded triangular with highest point at about a third of length, anterior margin broadly rounded, posterior end more pointed, dorsal margin only very weakly curved over the hinge, ventral side slightly concave. Surface of valves smooth. No lists recognisable on inner lamella of the juvenile valves. Central muscle scars paw-like, marginal pore channels straight and numerous.
Remarks. No adult valves were available for description. Adult valves are needed for a comprehensive description of this species.
Genus Paraeucypris Schneider in Mandelstam et al., 1957
Paraeucypris sp.
Figure 7c–f
Studied material. 802 (adults and juvenile).
Size. 0.86–1.5 mm.
Description. Valves elongated elliptical in lateral view, both ends well rounded, highest point well in front of mid-length, posterior part of right valves more slender than anterior one, dorsal margin along the hinge straight and distinctively inclined towards posterior, ventral margin slightly concave. Surface of valves smooth or fine pitted. Hinge with a simple groove in the left valve and a smooth bar in the right valve. Central muscle scars of the typical cypridid paw-like pattern.
Discussion and conclusion
Our list of ostracod taxa from the Zhada Basin contains at least eight species, several of them are already described for the Tibetan Plateau. Among them, the opportunistic and ubiquitous L. sinensis is the most abundant species. Leucocytherella sinensis is often observed together with the deeper lacustrine species ?L. dorsotuberosa and ?L. postilirata as it is known for recent faunas (Wrozyna et al. 2009; Akita et al. 2016). Kempf et al. (2009) list only five species from the Zhada Basin. One of them, Candona xizangensis Huang, 1982, was not found in our study. Adding C. xizangensis to our list results in a minimum of nine species, representing a low diversity for the studied area and time. Akita et al. (2016) found eleven species in the recent Tangra Yumco lake system, a number comparable to our count from the Zhada Basin. Considering that other investigated Cenozoic to modern lake basins of the Tibetan Plateau (Wrozyna et al. 2010; Mischke 2012; Alivernini et al. 2018a) with different salinities and depths have a similar low-diversity fauna, we assume the high altitude and the relative isolation of the Tibetan Plateau as the cause for the low diversity observed in the Zhada Basin.
The newly described species L. dangeloi sp. nov. is very interesting for the evolution of the genus Leucocytherella Huang, 1982, endemic to the Tibetan Plateau. All specimens of the genus described so far and studied by Fürstenberg et al. (2015) belong to L. sinensis Huang, 1982. The accompanying taxa of the species L. dangeloi sp. nov. from the Zhada Basin are, similar to the recent ostracod fauna of the Tibetan Plateau, mainly lacustrine species. Thus, the association of L. dangeloi sp. nov. with the lacustrine species points to a lacustrine habitat of the new species as well.
The species assemblage changes recorded in the sampled lake beds of the “South Zhada” section (Table 2) probably reflect changes in the depositional setting including more shallow and deltaic conditions or also deeper environments. However, combined sedimentological and geochemical analyses together with quantitative palaeoecological analysis of ostracod species assemblage data from the sediments of the Zhada Basin based on the presented taxonomical research are required to better understand the Miocene to Pleistocene environmental and climatic history of the western Tibetan Plateau.
References
Akita, L.G., P. Frenzel, N. Börner, J. Wang, and P. Peng. 2016. Distribution and ecology of the recent Ostracoda of the Tangra Yumco lake system, Southern Tibetan Plateau, China. Limnologica 59: 21–43.
Alivernini, M., Z. Lai, P. Frenzel, S. Fürstenberg, J. Wang, Y. Guo, P. Peng, T. Haberzettl, N. Börner, and S. Mischke. 2018a. Late Quaternary lake level changes of Taro Co and neighbouring lakes, southwestern Tibetan Plateau, based on OSL dating and ostracod analysis. Global and Planetary Change 166: 1–18.
Alivernini, M., L.G. Akita, M. Ahlborn, N. Börner, T. Haberzettl, T. Kasper, B. Plessen, P. Peng, A. Schwalb, J. Wang, and P. Frenzel. 2018b. Ostracod-based reconstruction of Late Quaternary lake level changes within the Tangra Yumco lake system (southern Tibetan Plateau). Journal of Quaternary Science 33: 713–720.
Baird, W. 1850. The natural history of the British Entomostraca, i–viii + 1–364. London: The Ray Society.
Boomer, I., D.J. Horne, and I.J. Slipper. 2003. The use of ostracods in palaeoenvironmental studies, or what can you do with an ostracod shell? The Paleontological Society Papers 9: 153–180.
Brady, S., and A.M. Norman. 1889. A monograph of the marine and freshwater Ostracoda of the North Atlantic and of North-Western Europe. Section I Podocopa. The Scientific Transactions of the Royal Dublin Society 4(2): 63–270.
Brady, S., and A.M. Norman. 1896. A monograph of the marine and freshwater Ostracoda of the North Atlantic and of North-Western Europe. Sections II-IV, Myodocopa, Cladocopa, and Platycopa. The Scientific Transactions of the Royal Dublin Society 4(2): 621–746.
Danielopol, D.L., J. Martens, and L.M. Casale. 1989. Revision of the genus Leucocythere Kaufmann, 1892 (Crustacea, Ostracoda, Limnocytheridae), with the description of a new species and two new tribes. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique 59: 63–64.
Frenzel, P., C. Wrozyna, M. Xie, L. Zhu, and A. Schwalb. 2010. Palaeowater depth estimation for a 600–year record from Nam Co (Tibet) using an ostracod–based transfer function. Quaternary International 218: 157–165.
Fürstenberg, S., P. Frenzel, P. Peng, K. Henkel, and C. Wrozyna. 2015. Phenotypical variation in Leucocytherella sinensis Huang, 1982 (Ostracoda): a new proxy for palaeosalinity in Tibetan lakes. Hydrobiologia 751: 55–72.
Guo, Y., L. Zhu, P. Frenzel, Q. Ma, J. Ju, P. Peng, J. Wang, and G. Daut. 2016. Holocene lake level fluctuations and environmental changes at Taro Co, southwestern Tibet, based on ostracod inferred water depth reconstruction. The Holocene 26: 29–43.
Jones, T.R. 1901. [a table]. On some Fossils of Wenlock Age from Mulde, near Klinteberg, Gotland, F. Chapman. Annals and Magazine of Natural History Series (7) 7: 146–147.
Horne, D.J. 2003. Key events in the radiation of the Ostracoda. In Bridging the gap: Trends in the Ostracode Biological and Geological Sciences, eds. L.E. Park, and A.J. Smith. The Paleontological Society Papers 9: 181–201.
Horne, D.J. 2007. A mutual temperature range method for Quaternary palaeoclimatic analysis using European nonmarine Ostracoda. Quaternary Science Reviews 26: 1398–1415.
Hou, Y., and Y. Gou. 2007. Fossil Ostracoda of China. Cytheracea and Cytherellidae, vol. 2. Beijing: Science Press. (in Chinese).
Hou, Y., Y. Gou, and D. Chen. 2002. Fossil Ostracoda of China. Superfamilies Cypridacea and Darwinulidacea, vol. 1. Beijing: Science Press. (in Chinese).
Huang, B. 1982. Ostracods from surface deposits of Recent lakes in Xizang. Acta Micropalaeontologica Sinica 2: 369–376. (in Chinese with English abstract).
Huang, B. 1987. Quaternary ostracod biogeographical provinces in China. Acta Palaeontologica Sinica 12: 117–119.
Kaufmann, A. 1892. Die Schweizerischen Cytheriden und ihre nächsten Verwandten. Revue Suisse de Zoologie 4: 313–384.
Kempf, O., P.M. Blisniuk, S. Wang, X. Fang, C. Wrozyna, and A. Schwalb. 2009. Sedimentology, sedimentary petrology, and paleoecology of the monsoon-driven, fluvio-lacustrine Zhada Basin, SW-Tibet. Sedimentary Geology 222: 27–41.
Klie, W. 1938. Krebstiere oder Crustacea 3: Ostracoda, Muschelkrebse. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise 34: 1–230.
Latreille, P.A. 1802. Histoire naturelle, générale et particulière, des crustacés et des insectes 3. Paris: F. Dufart.
Li, Y.F., Q.S. Zhang, B.Y. Li, and F.T. Liu. 1991. Late Pleistocene Ostracoda from Banggong Lake, Xizang and its palaeogeographic significance. Acta Micropalaeontologica Sinica 8: 57–64. (in Chinese with English Abstract).
Lu, F., Z. An, J. Dodson, X. Li, and H. Yan. 2019. A late Miocene ostracod record from the northeastern Tibetan Plateau. Journal of Paleolimnology 61: 297–312.
Lüttig, G. 1962. Zoologische und Paläontologische Ostracoden-Systematik. Paläontologische Zeitschrift 36: 154–184.
Mandelstam, N.I., G.F. Schneider, Z.V. Kuznetsova, and F.I. Kats. 1957. New genera of Ostracoda in the families Cypridae and Cytheridae. Ežegodnik Vsesojuznogo Paleontologičeskogo Obščestva 16: 166–192. (in Russian).
Martin, J.W., and G.E. Davis. 2001. An updated classification of the Recent Crustacea. Natural History Museum of Los Angeles County; Science Series 39: 1–132.
Mazzini, I., E. Gliozzi, G. Rossetti, and V. Pieri. 2014. The Ilyocypris puzzle: A multidisciplinary approach to the study of phenotypic variability. International Review of Hydrobiology 99(6): 395–408.
Meisch, C. 2000. Freshwater Ostracoda of Western and Central Europe. Heidelberg: Spektrum Akademischer Verlag.
Mischke, S. 2012. Quaternary ostracods from the Tibetan Plateau and their significance for environmental and climate-change studies. In Ostracoda as Proxies for Quaternary Climate Change, eds. D.J. Horne, J. Holmes, J. Rodriguez-Lazaro, and F. Viehberg. Developments in Quaternary Science 17: 263–279.
Mischke, S., Z. Sun, U. Herzschuh, Z. Qiao, and N. Sun. 2010. An ostracod-inferred large Middle Pleistocene freshwater lake in the presently hyper-arid Qaidam Basin (NW China). Quaternary International 218: 74–85.
Mischke, S., U. Herzschuh, Z. Sun, Z. Qiao, N. Sun, and A.M. Zander. 2006. Middle Pleistocene Ostracoda from a large freshwater lake in the presently dry Qaidam Basin (NW China). Journal of Micropalaeontology 25: 57–64.
Müller, G.W. 1894. Die Ostrakoden des Golfes von Neapel und der Angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel und der Angrenzenden Meeres-Abschnitte 21: 1–404.
Pang, J. 1985. On a new Ostracoda genus from Pleistocene in the pass of Kunlun Mountain, Qinghai-Xizang (Tibet) Plateau. Collection of Geology of the Qinghai–Xizang (Tibet) Plateau 16: 257–279. (in Chinese with English Abstract).
Peng, J.L. 1997. Ostracod assemblages and environmental changes during 13000–4500 a BP in Peiku Co, Tibet. Acta Micropalaeontologica Sinica 14: 239–254.
Saylor, J. 2008. The Late Miocene through modern evolution of the Zhada Basin, south-western Tibet. PhD thesis, The University of Arizona, Department of Geosciences.
Saylor, J., P. Decelles, and J. Quade. 2010a. Climate-driven environmental change in the Zhada Basin, southwestern Tibetan Plateau. Geosphere 6: 74–92.
Saylor, J., P. DeCelles, G. Gehrels, M. Murphy, R. Zhang, and P. Kapp. 2010b. Basin formation in the High Himalaya by arc-parallel extension and tectonic damming: Zhada Basin, southwestern Tibet. Tectonics 29(1): TC1004.
Song, B., J. Ji, C. Wang, and Y. Xu. 2017. Intensified aridity in the Qaidam Basin during the Middle Miocene: constraints from ostracod, stable isotope, and weathering records. Journal of Earth Science 54: 242–256.
Sun, Z. 1988. Tertiary Ostracode fauna from Qaidam Basin, NW China. Nanjing: Nanjing University Press. (in Chinese with English abstract).
Vávra, W. 1891. Monographie der Ostracoden Böhmens. Archiv für die naturwissenschaftliche Landesdurchforschung 8(3): 1–116.
Wang, W., J. Zhang, and B. Zhang. 2004. Structural and sedimentary features in Zanda Basin of Tibet. Acta Scientiarum Naturalium 40: 872–878.
Wang, S.F., P. Blisniuk, O. Kempf, X.M. Fang, F. Chun, and E. Wang. 2008. The basin-range system along the south segment of the Karakorum fault zone, Tibet. International Geology Review 50: 121–134.
Wrozyna, C., P. Frenzel, M. Xie, L. Zhu, and A. Schwalb. 2009. A taxonomical and ecological overview of Recent and Holocene ostracodes of the Nam Co region, southern Tibet. Quaternary Sciences 29: 665–677.
Wrozyna, C., P. Frenzel, P. Steeb, L. Zhu, R. Van Geldern, A. Mackensen, and A. Schwalb. 2010. Stable isotope and ostracode species assemblage evidence for lake level changes of Nam Co, southern Tibet, during the past 600 years. Quaternary International 212: 2–13.
Wu, K., J. Yu, and Q. Feng. 2011. Miocene-Pliocene Ostracoda assemblage and its geological significance in Eboliang Area, Qaidam Basin. Advances in Geosciences 1: 54–64.
Yang, L., Y. Fan, and W. Huang. 1982. Relation between ostracode distribution in surface deposits and water salt of Recent lakes in Xizang Plateau. Transactions of Oceanology and Limnology 1: 19–28. (in Chinese with English abstract).
Yang, F., Z. Sun, Z. Ma, and Y. Zhang. 1997. Quaternary ostracode zones and magnetostratigraphic profiles in the Qaidam Basin. Acta Micropalaeontologica Sinica 14: 378–390. (in Chinese with English abstract).
Zhang, P.X., B.Z. Zhang, and W.B. Yang. 1989. On the model of post-glacial palaeoclimatic fluctuation in Qinghai Lake region. Quaternary Sciences 9: 66–77. (in Chinese with English Abstract).
Zhang, P., B. Zhang, G. Qian, H. Li, and L. Xu. 1994. The study of paleoclimatic parameter of Qinghai Lake since Holocene. Quaternary Sciences 8: 225–238. (in Chinese with English Abstract).
Zhang, L., Z. Sun, Z. An, W. Liu, and X. Li. 2006. A preliminary distribution analysis on Ostracoda of different water bodies from Qinghai Lake area, NW China. Acta Micropalaeontologica Sinica 23: 425–436. (in Chinese with English Abstract).
Zhu, L., P. Peng, M. Xie, J. Wang, P. Frenzel, C. Wrozyna, and A. Schwalb. 2010. Ostracod-based environmental reconstruction over the last 8,400 years of Nam Co Lake on the Tibetan plateau. Hydrobiologia 648: 157–174.
Acknowledgements
Open Access funding provided by Projekt DEAL. We are indebted to Tim Jonas and Karsten Adler for help during fieldwork. Funding was provided by the German Research Foundation (Grants Mi 730/11+/16 and Fr 1489/4) within the Priority Program SPP 1372 TiP “Tibetan Plateau: Formation–Climate–Ecosystems” and by the Graduate Scholarship of Thuringia. Additional funding was received from the National Natural Science Foundation of China (41571189). We thank Ping Peng (Beijing) and Dada Yan (Shanghai) for translations of original ostracod descriptions in Chinese. We are indebted to the reviewers Finn Viehberg, Elsa Gliozzi and Marius Stoica for their constructive comments to improve the quality of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Mike Reich.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alivernini, M., Wang, J., Frenzel, P. et al. Mio–Pleistocene Ostracoda from the Zhada Basin (western Tibetan Plateau). PalZ 95, 37–54 (2021). https://doi.org/10.1007/s12542-020-00523-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-020-00523-w