Abstract
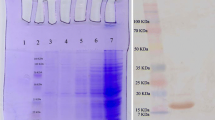
During the past two decades, tumor therapy based on monoclonal antibody has been found as a confident therapeutic approach in solid tumors and hematologic malignancies. Nanobodies are the smallest fragment of an antigen-binding domain in heavy chain-only antibody originated from the Camelidae family. Accordingly, they are being recently developed rapidly as diagnostic and therapeutic agents. In this regard, targeting of angiogenic factors like Placenta growth factor (PLGF) via nanobodies show a high effectiveness. In the current study, we developed a recombinant anti-PLGF bivalent nanobody based on the affinity enhancement mutant form of anti-PLGF nanobody to suppress the angiogenesis progression. Thereafter, the bivalent nanobody (bi-Nb) was cloned and then expressed into a bacterial system. Afterward, the purity was authorized using western blot assay and the affinity was assessed using ELISA. In this regard, proliferation, 3D capillary tube formation, and migration assays were employed as functional assays. The obtained data were analyzed using t-test and P < 0.05 was considered as statistically significant. The results indicate that the bivalent nanobody could inhibit proliferation, mobility, and formation of endothelial cell capillary-like structure. Moreover, the EC50 was estimated for endothelial cell’s proliferation and capillary tube’s formation to be about 100 ng/ml and 65 ng/ml, respectively. Migration of MCF-7 was inhibited as about 69%, rather than the control. Accumulation of data have shown that targeting of angiogenic factors like VEGF via monoclonal antibodies or nanobodies can be useful in the suppression of tumor progression. Also, the inhibition of PLGF with monoclonal antibody indicated that it is significant in angiogenesis suppression. However, due to intrinsic properties of nanobodies, they are suggested to be used. Since the small size is rapidly removed through liver or kidney system, so it is important to use bivalent or polymeric forms for extending the half-life. Our findings indicated that the inhibition of PLGF can prevent growth and proliferation of endothelial cells and tumor cells through the bivalent nanobody. So, it is suggested as a novel therapeutic agent for angiogenesis suppression.






Similar content being viewed by others
Abbreviations
- AMD:
-
Age-related macular degeneration
- Ang:
-
Angiopoietin
- bi-Nb:
-
Bivalent nanobody
- bi-Nb-PLGF-pHEN6c:
-
Anti-PLGF bivalent nanobody-pHEN6c
- CML:
-
Chronic myeloid leukemia
- DMEM:
-
Dulbecco’s modified eagle medium
- EGF:
-
Epidermal growth factor
- ELISA:
-
Enzyme-linked immunosorbent assay
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia-inducible factor-1 alpha
- HRP-conjugated antibody:
-
Horse radish peroxidase-conjugated antibody
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- PDGF:
-
Platelet-derived growth factor
- PI3K:
-
Phosphatidylinositol-3 kinase
- PLGF:
-
Placenta growth factor
- PTM:
-
Post-translational modification
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
- VHH:
-
Variable domain of the heavy chain of a heavy chain antibody
- VWF:
-
Von Willebrand factor
- MCF-7:
-
Michigan Cancer Foundation-7(a breast cancer cell line)
- MTT:
-
3, 4, 5-Dimethylthiazol-2-yl-2–5-diphenyltetrazolium bromide
- Ni–NTA:
-
Nickel ion-nitrilotriacetic acid
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- IgA:
-
Immunoglobulin A
- IgG:
-
Immunoglobulin G
- IPTG:
-
Isopropyl β-D-1 thiogalactopyranoside
- TB medium:
-
Terrific broth medium
- TMB:
-
3, 3′, 5, 5′-Tetramethyl benzidine
- TNF-α:
-
Tumor necrosis factor alpha
- HUVEC:
-
Human umbilical vein endothelial cell
- DAB:
-
Diaminobenzidine
References
Tetzlaff, F., & Fischer, A. (2018). Human endothelial cell spheroid-based sprouting angiogenesis assay in collagen. Bio-Protocol, 8(17), e2995.
Nishida, N., et al. (2006). Angiogenesis in cancer. Vascular Health and Risk Management, 2(3), 213–219.
De Falco, S. (2012). The discovery of placenta growth factor and its biological activity. Experimental & Molecular Medicine, 44, 1.
Apicella, I., et al. (2018). Full functional knockout of placental growth factor by knockin with an inactive variant able to heterodimerize with VEGF-A. Cell Reports, 23(12), 3635–3646.
Tarallo, V., et al. (2010). A placental growth factor variant unable to recognize vascular endothelial growth factor (VEGF) receptor-1 inhibits VEGF-dependent tumor angiogenesis via heterodimerization. Cancer Research, 70(5), 1804–1813.
Lazzara, F., et al. (2019). Aflibercept regulates retinal inflammation elicited by high glucose via the PlGF/ERK pathway. Biochemical Pharmacology, 168, 341–351.
Lazzara, F., et al. (2020). Stabilization of HIF-1α in human retinal endothelial cells modulates expression of miRNAs and proangiogenic growth factors. Frontiers in Pharmacology, 11(1063), e01063.
Albonici, L., et al. (2019). Multifaceted role of the placental growth factor (PlGF) in the antitumor immune response and cancer progression. International Journal of Molecular Sciences, 20(12), 2970.
Pagani, E., et al. (2016). Placenta growth factor and neuropilin-1 collaborate in promoting melanoma aggressiveness. International Journal of Oncology, 48(4), 1581–1589.
Martinsson-Niskanen, T., et al. (2011). Monoclonal antibody TB-403: A first-in-human, phase I, double-blind, dose escalation study directed against placental growth factor in healthy male subjects. Clinical Therapeutics, 33(9), 1142–1149.
Dewerchin, M., & Carmeliet, P. (2012). PlGF: A multitasking cytokine with disease-restricted activity. Cold Spring Harbor Perspectives in Medicine, 2(8), a011056.
Arbabi-Ghahroudi, M. (2017). Camelid single-domain antibodies: Historical perspective and future outlook. Frontiers in Immunology, 8, 01589.
Leow, C. H., et al. (2018). The development of single domain antibodies for diagnostic and therapeutic applications. Antibody Engineering, 175.
Bannas, P., Hambach, J., & Koch-Nolte, F. (2017). Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics. Frontiers in Immunology, 8, 01603.
Van Audenhove, I., & Gettemans, J. (2016). Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. Ebiomedicine, 8, 40–48.
Hu, Y. Z., Liu, C. X., & Muyldermans, S. (2017). Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Frontiers in Immunology, 8, 01442.
Kijanka, M., et al. (2015). Nanobody-based cancer therapy of solid tumors. Nanomedicine, 10(1), 161–174.
Kontermann, R. E. (2009). Strategies to extend plasma half-lives of recombinant antibodies. Biodrugs, 23(2), 93–109.
Klein, J. S., et al. (2014). Design and characterization of structured protein linkers with differing flexibilities. Protein Engineering Design & Selection, 27(10), 325–330.
Chen, X. Y., Zaro, J. L., & Shen, W. C. (2013). Fusion protein linkers: Property, design and functionality. Advanced Drug Delivery Reviews, 65(10), 1357–1369.
Movahedi, K., et al. (2012). Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Research, 72(16), 4165–4177.
Scully, M., et al. (2019). Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. New England Journal of Medicine, 380(4), 335–346.
Fleischmann, R., et al. (2011). A multiple ascending dose/proof of concept study of ATN-103 (ozoralizumab) in rheumatoid arthritis subjects on a background of methotrexate. Arthritis and Rheumatism, 63(10), S1033–S1034.
Ebrahimi, Z., et al. (2018). Rational affinity enhancement of fragmented antibody by ligand-based affinity improvement approach. Biochemical and Biophysical Research Communications, 506(3), 653–659.
Arezumand, R., et al. (2016). Identification and characterization of a novel nanobody against human placental growth factor to modulate angiogenesis. Molecular Immunology, 78, 183–192.
Beatty, J. D., Beatty, B. G., & Vlahos, W. G. (1987). Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. Journal of Immunological Methods, 100(1–2), 173–179.
Cabral, T., et al. (2017). Retinal and choroidal angiogenesis: A review of new targets. International Journal of Retina and Vitreous, 3(1), 31.
Kong, D. H., et al. (2017). A review of anti-angiogenic targets for monoclonal antibody cancer therapy. International Journal of Molecular Science, 18(8), 1786.
Ceci, C., et al. (2020). Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: Experimental evidence in different metastatic cancer models. International Journal of Molecular Science, 21(4), 1388.
Giurdanella, G., et al. (2015). Aflibercept, bevacizumab and ranibizumab prevent glucose-induced damage in human retinal pericytes in vitro, through a PLA2/COX-2/VEGF-A pathway. Biochemical Pharmacology, 96(3), 278–287.
Spadiut, O., et al. (2014). Microbials for the production of monoclonal antibodies and antibody fragments. Trends in Biotechnology, 32(1), 54–60.
Sadeghi, A., et al. (2019). Development of a mono-specific anti-VEGF bivalent nanobody with extended plasma half-life for treatment of pathologic neovascularization. Drug Testing and Analysis, 12, 92.
Acknowledgements
This project was financially supported by North Khorasan University of Medical Sciences .and National Institute for medical research development (Nimad).
Funding
This project was financially supported by North Khorasan University of Medical Sciences, Bojnurd, Iran.
Author information
Authors and Affiliations
Contributions
RA: management the research group and analysis the result and writing the manuscript. AN: perform the assays and initial analysis and writing the manuscript. KM: help to performing the angiogenesis assays. AHK: writing the draft of manuscript. HNAA: writing the draft of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikooharf, A., Arezumand, R., Mansouri, K. et al. Development of a Recombinant Monospecific Anti-PLGF Bivalent Nanobody and Evaluation of it in Angiogenesis Modulation. Mol Biotechnol 62, 580–588 (2020). https://doi.org/10.1007/s12033-020-00275-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-020-00275-7