Abstract
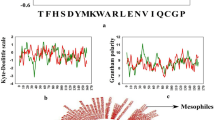
Hyperthermophiles, a subset of prokaryotes that thrive in adverse temperatures, potentially utilize the protein molecular biosystem for maintaining thermostability in a wide range of temperatures. Recent studies revealed that these organisms have smaller proportions of intrinsically disordered proteins. In this study, we performed sequence and structural analysis to investigate the maintenance of protein conformation and their stability at different temperatures. The sequence analysis reveals the higher proportion of charged amino acids are responsible for preventing the helix formation and, hence, become disordered regions. For structural analysis, we chose shikimate dehydrogenase from four species, namely Listeria monocytogenes, Escherichia coli, Thermus thermophilus, and Methanopyrus kandleri, and evaluated the protein adaptation at 283 K, 300 K, and 395 K temperatures. From this investigation, we found more residues of shikimate dehydrogenase prefer an order-to-disorder transition at 395 K only for hyperthermophilic species. The solvent-accessible surface area (SASA) and hydrogen-bond analysis revealed that the tertiary conformation and the number of hydrogen bonds for hyperthermophilic shikimate dehydrogenase are highly preserved at 395 K, compared to 300 K. Our simulation results conjointly provide shikimate dehydrogenase of hyperthermophile which resists high temperatures through stronger protein tertiary conformations.






Similar content being viewed by others
References
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181:223–230. https://doi.org/10.1126/science.181.4096.223
Babu MM (2016) The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochem Soc Trans 44:1185. https://doi.org/10.1042/BST20160172
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350. https://doi.org/10.1093/bioinformatics/btq662
Berman HM, Westbrook J, Feng Z et al (2000) The protein data bank. Nucleic Acids Res 28:235–242
Burra PV, Kalmar L, Tompa P (2010) Reduction in structural disorder and functional complexity in the thermal adaptation of prokaryotes. PLoS ONE 5:e12069. https://doi.org/10.1371/journal.pone.0012069
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519. https://doi.org/10.1002/pro.5560020916
Dill KA, Ozkan SB, Shell MS, Weikl TR (2008) The protein folding problem. Annu Rev Biophys 37:289–316. https://doi.org/10.1146/annurev.biophys.37.092707.153558
Dosztányi Z, Csizmok V, Tompa P, Simon I (2005) IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 21:3433–3434. https://doi.org/10.1093/bioinformatics/bti541
Du X, Sang P, Xia Y-L et al (2017) Comparative thermal unfolding study of psychrophilic and mesophilic subtilisin-like serine proteases by molecular dynamics simulations. J Biomol Struct Dyn 35:1500–1517. https://doi.org/10.1080/07391102.2016.1188155
Dutoit R, de Ruyck J, Durisotti V et al (2008) Overexpression, physicochemical characterization, and modeling of a hyperthermophilic pyrococcus furiosus type 2 IPP isomerase. Proteins 71:1699–1707. https://doi.org/10.1002/prot.21863
Eisenberg D, Lüthy R, Bowie JU (1997) VERIFY3D: assessment of protein models with three-dimensional profiles. Methods in enzymology. Academic Press, Cambridge, pp 396–404
Fiser A (2010) Template-based protein structure modeling. Methods Mol Biol 673:73–94. https://doi.org/10.1007/978-1-60761-842-3_6
Forman-Kay JD, Mittag T (2013) From sequence and forces to structure, function and evolution of intrinsically disordered proteins. Structure 21:1492–1499. https://doi.org/10.1016/j.str.2013.08.001
Fukuchi S, Yoshimune K, Wakayama M et al (2003) Unique amino acid composition of proteins in halophilic bacteria. J Mol Biol 327:347–357. https://doi.org/10.1016/s0022-2836(03)00150-5
Gasteiger E, Gattiker A, Hoogland C et al (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784. https://doi.org/10.1093/nar/gkg563
Givol D, Lorenzo FD, Goldberger RF, Anfinsen CB (1965) Disulfide interchange and the three-dimensional structure of proteins. Proc Natl Acad Sci USA 53:676–684
Jaenicke R (1991) Protein stability and molecular adaptation to extreme conditions. Eur J Biochem 202:715–728
Jaenicke R, Závodszky P (1990) Proteins under extreme physical conditions. FEBS Lett 268:344–349
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577–2637. https://doi.org/10.1002/bip.360221211
Karpen ME, de Haseth PL, Neet KE (1989) Comparing short protein substructures by a method based on backbone torsion angles. Proteins 6:155–167. https://doi.org/10.1002/prot.340060206
Khan S, Farooq U, Kurnikova M (2016) Exploring protein stability by comparative molecular dynamics simulations of homologous hyperthermophilic, mesophilic, and psychrophilic proteins. J Chem Inf Model 56:2129–2139. https://doi.org/10.1021/acs.jcim.6b00305
Lam SD, Das S, Sillitoe I, Orengo C (2017) An overview of comparative modelling and resources dedicated to large-scale modelling of genome sequences. Acta Crystallogr D Struct Biol 73:628–640. https://doi.org/10.1107/S2059798317008920
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291. https://doi.org/10.1107/S0021889892009944
Lee K-J (2012) Molecular dynamics simulations of a hyperthermophilic and a mesophilic protein L30e. J Chem Inf Model 52:7–15. https://doi.org/10.1021/ci200184y
Lieutaud P, Ferron F, Uversky AV et al (2016) How disordered is my protein and what is its disorder for? A guide through the “dark side” of the protein universe. Intrinsically Disord Proteins. https://doi.org/10.1080/21690707.2016.1259708
Mamat B, Roth A, Grimm C et al (2002) Crystal structures and enzymatic properties of three formyltransferases from archaea: environmental adaptation and evolutionary relationship. Protein Sci 11:2168–2178. https://doi.org/10.1110/ps.0211002
Mir R, Jallu S, Singh TP (2015) The shikimate pathway: review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit Rev Microbiol 41:172–189. https://doi.org/10.3109/1040841X.2013.813901
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Pronk S, Páll S, Schulz R et al (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29:845–854. https://doi.org/10.1093/bioinformatics/btt055
Razvi A, Scholtz JM (2006) Lessons in stability from thermophilic proteins. Protein Sci 15:1569–1578. https://doi.org/10.1110/ps.062130306
Rost B, Yachdav G, Liu J (2004) The PredictProtein server. Nucleic Acids Res 32:W321. https://doi.org/10.1093/nar/gkh377
Sang P, Yang Q, Du X et al (2016) Effect of the solvent temperatures on dynamics of serine protease proteinase K. Int J Mol Sci. https://doi.org/10.3390/ijms17020254
UniProt Consortium T (2018) UniProt: the universal protein knowledgebase. Nucleic Acids Res 46:2699. https://doi.org/10.1093/nar/gky092
Vicedo E, Schlessinger A, Rost B (2015) Environmental pressure may change the composition protein disorder in prokaryotes. PLoS ONE. https://doi.org/10.1371/journal.pone.0133990
Wallner B, Elofsson A (2003) Can correct protein models be identified? Protein Sci 12:1073–1086. https://doi.org/10.1110/ps.0236803
Webb B, Sali A (2016) Comparative protein structure modeling using MODELLER. Curr Protoc Bioinf 54:561–5637. https://doi.org/10.1002/cpbi.3
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35:W407–410. https://doi.org/10.1093/nar/gkm290
Xu D, Zhang Y (2011) Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys J 101:2525–2534. https://doi.org/10.1016/j.bpj.2011.10.024
Xue B, Williams RW, Oldfield CJ et al (2010) Archaic chaos: intrinsically disordered proteins in Archaea. BMC Syst Biol 4:S1. https://doi.org/10.1186/1752-0509-4-S1-S1
Yachdav G, Kloppmann E, Kajan L et al (2014) PredictProtein—an open resource for online prediction of protein structural and functional features. Nucleic Acids Res 42:W337. https://doi.org/10.1093/nar/gku366
Yang C, Jang S, Pak Y (2014) A fully atomistic computer simulation study of cold denaturation of a β-hairpin. Nat Commun 5:5773. https://doi.org/10.1038/ncomms6773
Ye S, von Delft F, Brooun A et al (2003) The crystal structure of shikimate dehydrogenase (AroE) reveals a unique NADPH binding mode. J Bacteriol 185:4144–4151. https://doi.org/10.1128/JB.185.14.4144-4151.2003
Yutani K, Matsuura Y, Naitow H, Joti Y (2018) Ion-ion interactions in the denatured state contribute to the stabilization of CutA1 proteins. Sci Rep 8:7613. https://doi.org/10.1038/s41598-018-25825-7
Zhang X, Meining W, Fischer M et al (2001) X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophile Aquifex aeolicus at 1.6 A resolution: determinants of thermostability revealed from structural comparisons. J Mol Biol 306:1099–1114. https://doi.org/10.1006/jmbi.2000.4435
Acknowledgements
The authors would like to acknowledge the management of Vellore Institute of Technology, Vellore for providing the facilities and encouragement to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by M. Moracci.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
792_2020_1198_MOESM1_ESM.png
Supplementary file1 Supplementary figure 1: (A) Multiple sequence alignment of AroE_Ecoli, AroE_Lismo, Ar-oE_Thet8 and AroE_Metka. (B) Structural superimposition of AroE protein. Color scheme: Green—Lismo, Blue—Thet8, Magneta—Ecoli and Red—Metka. (C) Table describing structural similarity score and Root Mean Square Devi-ation score of backbone (RMSD) (PNG 320 kb)
792_2020_1198_MOESM2_ESM.png
Supplementary file2 Supplementary figure 2: Number of hydrogen bonds. X-axis represents species name with temper-ature. Y-axis represents the number of hydrogen bonds. Color scheme: Red—300 K, Blue—283 K and Cyan—395 K (PNG 39 kb)
792_2020_1198_MOESM3_ESM.tif
Supplementary file3 Supplementary figure 3: Average bond length of hydrogen bond for each snapshots during the simulation. X-axis represents the name of the species and temperature. Y-axis represents the average hydrogen bond length (TIF 3281 kb)
Rights and permissions
About this article
Cite this article
Kamalesh, D., Nair, A., Sreeshma, J. et al. Statistical and molecular dynamics (MD) simulation approach to investigate the role of intrinsically disordered regions of shikimate dehydrogenase in microorganisms surviving at different temperatures. Extremophiles 24, 831–842 (2020). https://doi.org/10.1007/s00792-020-01198-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-020-01198-6