Abstract
Almost a half century of research has elaborated the discoveries of the central mechanisms governing the analgesic responses of opiates, including their receptors, endogenous peptides, genes and their putative spinal and supraspinal sites of action. One of the central tenets of “gate-control theories of pain” was the activation of descending supraspinal sites by opiate drugs and opioid peptides thereby controlling further noxious input. This review in the Special Issue dedicated to the research of Dr. Gavril Pasternak indicates his contributions to the understanding of supraspinal mediation of opioid analgesic action within the context of the large body of work over this period. This review will examine (a) the relevant supraspinal sites mediating opioid analgesia, (b) the opioid receptor subtypes and opioid peptides involved, (c) supraspinal site analgesic interactions and their underlying neurophysiology, (d) molecular (particularly AS) tools identifying opioid receptor actions, and (e) relevant physiological variables affecting site-specific opioid analgesia. This review will build on classic initial studies, specify the contributions that Gavril Pasternak and his colleagues did in this specific area, and follow through with studies up to the present.

Similar content being viewed by others
Abbreviations
- APV:
-
dl-2-Amino-5-phosphono-valerate
- AS:
-
Antisense
- BEND:
-
Beta-endorphin
- BFNA:
-
Beta-funaltrexamine
- Bupr:
-
Buprenorphine
- CCK:
-
Cholecystokinin
- DADL:
-
d-Ala2, d-Leu5-enkephalin
- DAMGO:
-
d-Ala2, MePhe4, Gly(ol)5-enkephalin
- Delt:
-
d-Ala2, Glu4 deltorphin
- DOR-1:
-
Delta opioid receptor clone
- DPDPE:
-
d-Pen2, d-Pen5-enkephalin
- DSLET:
-
d-Ser2, Leu5-enkephalin-Thr6
- EAA:
-
Excitatory amino acid
- EKC:
-
Ethylketocyclazocine
- GI:
-
Gastrointestinal
- GPCR:
-
G-protein coupled receptor
- IBNtxA:
-
39-Iodobenzoyl-6b-naltrexamide
- icv:
-
Intracerebroventricular
- it:
-
Intrathecal
- KO:
-
Knockout
- KOR-1:
-
Kappa-1 opioid receptor clone
- KOR-3:
-
Kappa-3 opioid receptor clone
- LC:
-
Locus coeruleus
- MA:
-
Mercaptoacetate
- mPFC:
-
Medial prefrontal cortex
- MOR-1:
-
Mu opioid receptor clone
- MPOA:
-
Medial preoptic hypothalamus
- M3G:
-
Morphine-3 glucuronide
- M6G:
-
Morphine-6 glucuronide
- NAC:
-
Nucleus accumbens
- NalBzoH:
-
Naloxone benzoylhydrazone
- NBNI:
-
Nor-binaltorphimine
- NE:
-
Norepinephrine
- NMDA:
-
N-methyl-d-aspartate
- NPY:
-
Neuropeptide Y
- NRGC:
-
Nucleus reticularis gigantocellularis
- NRM:
-
Nucleus raphe magnus
- NTI:
-
Naltrindole
- NTII:
-
Naltrindole isothiocyanate
- ODNs:
-
Oligodeoxynucleotides
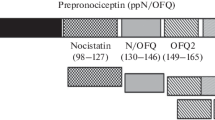
- OFQ/N:
-
Orphanin/FQ
- PCB:
-
1-(p-Chlorobenzoyl)-piperazine-2,3-dicarboxylate
- PPENK:
-
Prepro-enkephalin
- ppOFQ/N:
-
Prepro-orphanin/FQ/nociception
- SPA:
-
Stimulation-produced analgesia
- TP:
-
Testosterone propionate
- TRH:
-
Thyrotropin-releasing hormone
- VMH:
-
Ventromedial hypothalamus
- vlPAG:
-
Ventrolateral periaqueductal gray
- VTA:
-
Ventral tegmental area
- 2DG:
-
2-Deoxy-d-glucose
- 5HT:
-
Serotonin
References
Abbadie C, Rossi GC, Orciuolo A, Zadina JE, Pasternak GW (2002) Anatomical and functional correlation of the endomorphins with mu opioid receptor splice variants: endomorphins and mu opioid receptor splice variants. Eur J Neurosci 16:1075–1082
Abbadie C, Pan YX, Pasternak GW (2004) Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in mouse brain. Neuroscience 127:419–430. https://doi.org/10.1016/j.neuroscience.2004.03.033
Abbott FV, Palmour RM (1988) Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life Sci 43:1685–1695. https://doi.org/10.1016/0024-3205(88)90479-1
Abols IA, Basbaum AI (1981) Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain-medullary interactions in the modulation of pain. J Comp Neurol 201:285–297
Aimone L, Gebhart G (1986) Stimulation-produced spinal inhibition from the midbrain in the rat is mediated by an excitatory amino acid neurotransmitter in the medial medulla. J Neurosci 6:1803–1813
Akaike A, Shibata T, Satoh M, Takagi H (1978) Analgesia induced by microinjection of morphine into, and electrical stimulation of, the nucleus reticularis paragigantocellularis of rat medulla oblongata. Neuropharmacology 17:775–778
Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM (1984) Endogenous opioids: biology and function. Annu Rev Neurosci 7:223–255
Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo C-O, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR (2015) Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87:1063–1077. https://doi.org/10.1016/j.neuron.2015.08.019
Al-Rodhan N, Chipkin R, Yaksh TL (1990) The antinociceptive effects of SCH-32615, a neutral endopeptidase (enkephalinase) inhibitor, microinjected into the periaqueductal, ventral medulla and amygdala. Brain Res 520:123–130
Appelbaum BD, Holtzman SG (1984) Characterization of stress-induced potentiation of opioid effects in the rat. J Pharmacol Exp Ther 231:555–565
Appelbaum BD, Holtzman SG (1985) Restraint stress enhances morphine-induced analgesia in the rat without changing apparent affinity of receptor. Life Sci 36:1069–1074. https://doi.org/10.1016/0024-3205(85)90492-8
Arjune D, Bodnar RJ (1990) Suppression of nocturnal, palatable and glucoprivic intake in rats by the κ opioid antagonist, nor-binaltorphamine. Brain Res 534:313–316
Arjune D, Standifer KM, Pasternak GW, Bodnar RJ (1990) Reduction by central ß-funaltrexamine of food intake in rats under freely-feeding, deprivation and glucoprivic conditions. Brain Res 535:101–109
Arjune D, Bowen WD, Bodnar RJ (1991) Ingestive behavior following central [D-Ala2, Leu5, Cys6]-enkephalin (DALCE), a short-acting agonist and long-acting antagonist at the delta opioid receptor. Pharmacol Biochem Behav 39:429–436
Aston-Jones G, Ennis M, Pieribone V, Nickell W, Shipley M (1986) The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science 234:734–737
Atweh SF, Kuhar MJ (1977a) Autoradiographic localization of opiate receptors in rat brain. I. Spinal cord and lower medulla. Brain Res 124:53–67
Atweh SF, Kuhar MJ (1977b) Autoradiographic localization of opiate receptors in rat brain. II. The brain stem. Brain Res 129:1–12
Azami J, Wright DM, Roberts MHT (1981) Effects of morphine and naloxone on the responses to noxious stimulation of neurones in the nucleus reticularis paragigantocellularis. Neuropharmacology 20:869–876
Azami J, Llewelyn MB, Roberts MHT (1982) The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain 12:229–246
Baamonde AI, Hidalgo A, Andrés-Trelles F (1989) Sex-related differences in the effects of morphine and stress on visceral pain. Neuropharmacology 28:967–970
Badillo-Martinez D, Kirchgessner AL, Butler PD, Bodnar RJ (1984a) Monosodium glutamate and analgesia induced by morphine. Test-specific effects. Neuropharmacology 23:1141–1149. https://doi.org/10.1016/0028-3908(84)90231-4
Badillo-Martinez D, Nicotera N, Butler PD, Kirchgessner AL, Bodnar RJ (1984b) Impairments in analgesic, hypothermic, and glucoprivic stress responses following neonatal monosodium glutamate. Neuroendocrinology 38:438–446. https://doi.org/10.1159/000123932
Bajic D, Proudfit HK (1999) Projections of neurons in the periaqueductal gray to pontine and medullary catecholamine cell groups involved in the modulation of nociception. J Comp Neurol 405:359–379
Baliki MN, Geha PY, Fields HL, Apkarian AV (2010) Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66:149–160. https://doi.org/10.1016/j.neuron.2010.03.002
Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV (2013) Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci 33:16383–16393. https://doi.org/10.1523/JNEUROSCI.1731-13.2013
Bandler R, Shipley MT (1994) Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 17:379–389
Banerjee P, Chatterjee TK, Ghosh JJ (1983) Ovarian steroids and modulation of morphine-induced analgesia and catalepsy in female rats. Eur J Pharmacol 96:291–294
Barbaro NM, Hammond DL, Fields HL (1985) Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res 343:223–229
Barbaro NM, Heinricher MM, Fields HL (1986) Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res 366:203–210
Barr GA, Wang S (2013) Analgesia induced by localized injection of opiate peptides into the brain of infant rats. Eur J Pain 17:676–691. https://doi.org/10.1002/j.1532-2149.2012.00245.x
Bartok RE, Craft RM (1997) Sex differences in opioid antinociception. J Pharmacol Exp Ther 282:769–778
Basbaum AI, Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7:309–338
Beatty WW, Beatty PA (1970) Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol 73:446–455
Beczkowska IW, Bodnar RJ (1991) Mediation of insulin hyperphagia by specific central opiate receptor antagonists. Brain Res 547:315–318. https://doi.org/10.1016/0006-8993(91)90977-4
Behbehani MM, Fields HL (1979) Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res 170:85–93
Beitz A (1982a) The sites of origin brain stem neurotensin and serotonin projections to the rodent nucleus raphe magnus. J Neurosci 2:829–842
Beitz AJ (1982b) The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience 7:133–159
Beitz AJ (1982c) The nuclei of origin of brain stem enkephalin and substance P projections to the rodent nucleus raphe magnus. Neuroscience 7:2753–2768
Beitz AJ (1990) Relationship of glutamate and aspartate to the periaqueductal gray-raphe magnus projection: analysis using immunocytochemistry and microdialysis. J Histochem Cytochem 38:1755–1765
Beitz AJ (1995) Periaqueductal gray. In: Paxinos G (ed) The rat nervous system, vol xvii, 2nd edn. Academic Press, San Diego, pp 173–182
Beitz AJ, Mullett MA, Weiner LL (1983) The periaqueductal gray projections to the rat spinal trigeminal, raphe magnus, gigantocellular pars alpha and paragigantocellular nuclei arise from separate neurons. Brain Res 288:307–314
Bhargava HN, Way EL (1972) Acetylcholinesterase inhibition and morphine effects in morphine tolerant and dependent mice. J Pharmacol Exp Ther 183:31–40
Bilsky EJ, Bernstein RN, Pasternak GW, Hruby VJ, Patel D, Porreca F, Lai J (1994) Selective inhibition of [D-ALA2, GLU4]deltrophin antinociception by supraspinal, but not spinal, administration of an antisense oligodeoxynucleotide to an opioid delta receptor. Life Sci 55:PL37–PL43
Bobeck EN, McNeal AL, Morgan MM (2009) Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain 147:210–216
Bodnar RJ (1990) Effects of opioid peptides on peripheral stimulation and “stress”-induced analgesia in animals. Crit Rev Neurobiol 6:39–49
Bodnar RJ (2004) Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides 25:697–725
Bodnar RJ (2015) Endogenous opioids and feeding behavior: a decade of further progress (2004-2014). A Festschrift to Dr. Abba Kastin. Peptides 72:20–33. https://doi.org/10.1016/j.peptides.2015.03.019
Bodnar RJ (2019) Endogenous opioid modulation of food intake and body weight: implications for opioid influences upon motivation and addiction. Peptides 116:42–62
Bodnar RJ, Kelly DD, Spiaggia A, Ehrenberg C, Glusman M (1978a) Dose-dependent reductions by naloxone of analgesia induced by cold-water stress. Pharmacol Biochem Behav 8:667–672
Bodnar RJ, Kelly DD, Steiner SS, Glusman M (1978b) Stress-produced analgesia and morphine-produced analgesia: lack of cross-tolerance. Pharmacol Biochem Behav 8:661–666
Bodnar RJ, Glusman M, Brutus M, Spiaggia A, Kelly DD (1979a) Analgesia induced by cold-water stress: attenuation following hypophysectomy. Physiol Behav 23:53–62
Bodnar RJ, Kelly DD, Mansour A, Murray G (1979b) Differential effects of hypophysectomy upon analgesia induced by two glucoprivic stressors and morphine. Pharmacol Biochem Behav 11:303–308
Bodnar RJ, Abrams GM, Zimmerman EA, Krieger DT, Nicholson G, Kizer JS (1980a) Neonatal monosodium glutamate. Neuroendocrinology 30:280–284
Bodnar RJ, Kelly DD, Brutus M, Glusman M (1980b) Stress-induced analgesia: neural and hormonal determinants. Neurosci Biobehav Rev 4:87–100. https://doi.org/10.1016/0149-7634(80)90028-7
Bodnar RJ, Zimmerman EA, Nilaver G, Mansour A, Thomas LW, Kelly DD, Glusman M (1980c) Dissociation of cold-water swim and morphine analgesia in Brattleboro rats with diabetes insipidus. Life Sci 26:1581–1590
Bodnar RJ, Sharpless NS, Kordower JH, Potegal M, Barr GA (1982) Analgesic responses following adrenal demedullation and peripheral catecholamine depletion. Physiol Behav 29:1105–1109
Bodnar RJ, Portzline T, Nilaver G (1985) Differential alterations in opioid analgesia following neonatal monosodium glutamate treatment. Brain Res Bull 15:299–305
Bodnar RJ, Williams CL, Lee SJ, Pasternak GW (1988) Role of μ1-opiate receptors in supraspinal opiate analgesia: a microinjection study. Brain Res 447:25–34
Bodnar RJ, Paul D, Pasternak GW (1990a) Proglumide selectively potentiates supraspinal μ1 opioid analgesia in mice. Neuropharmacology 29:507–510
Bodnar RJ, Paul D, Rosenblum M, Liu L, Pasternak GW (1990b) Blockade of morphine analgesia by both pertussis and cholera toxins in the periaqueductal gray and locus coeruleus. Brain Res 529:324–328
Bodnar R, Paul D, Pasternak GW (1991) Synergistic analgesic interactions between the periaqueductal gray and the locus coeruleus. Brain Res 558:224–230
Boschi G, Desiles M, Reny V, Rips R, Wrigglesworth S (1983) Antinociceptive properties of thyrotropin releasing hormone in mice: comparison with morphine. Br J Pharmacol 79:85–92
Boyer JS, Morgan MM, Craft RM (1998) Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res 796(1–2):315–318. https://doi.org/10.1016/S0006-8993(98)00353-9
Brodie MS, Proudfit HK (1984) Hypoalgesia induced by the local injection of carbachol into the nucleus raphe magnus. Brain Res 291:337–342. https://doi.org/10.1016/0006-8993(84)91266-6
Brodie MS, Proudfit HK (1986) Antinociception induced by local injections of carbachol into the nucleus raphe magnus in rats: alteration by intrathecal injection of monoaminergic antagonists. Brain Res 371:70–79. https://doi.org/10.1016/0006-8993(86)90811-5
Brooks AI, Standifer KM, Rossi GC, Mathis JP, Pasternak GW (1996) Characterizing kappa3 opioid receptors with a selective monoclonal antibody. Synapse 22:247–252
Brown GP, Yang K, King MA, Rossi GC, Leventhal L, Chang A, Pasternak WG (1997) 3-Methoxynaltrexone, a selective heroin/morphine-6β-glucuronide antagonist. FEBS Lett 412:35–38
Brown TG, Xu J, Hurd YL, Pan YX (2020) Dysregulated expression of the alternatively spliced variant mRNAs of the mu opioid receptor gene, OPRM1, in the medial prefrontal cortex of male human heroin abusers and heroin self-administering male rats. J Neurosci Res. https://doi.org/10.1002/jnr.24640
Brownstein MJ, Palkovits M, Saavedra JM, Bassiri RM, Utiger RD (1974) Thyrotropin-releasing hormone in specific nuclei of rat. Brain Sci 185:267–269
Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a μ, δ or κ opioid receptor type. FEBS Lett 347:284–288
Burdick K et al (1998) Antisense mapping of opioid receptor clones: effects upon 2-deoxy-d-glucose-induced hyperphagia. Brain Res 794:359–363
Cameron AA, Khan IA, Westlund KN, Willis WD (1995) The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol 351:585–601
Candido J, Lutfy K, Billings B, Sierra V, Duttaroy A, Inturrisi CE, Yoburn BC (1992) Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol Biochem Behav 42:685–692
Cannon JT, Prieto GJ, Lee A, Liebeskind JC (1982) Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res 243:315–321
Cassel D, Selinger Z (1977) Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci 74:3307–3311
Castro DC, Berridge KC (2014) Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci 34:4239–4250. https://doi.org/10.1523/JNEUROSCI.4458-13.2014
Castro DC, Bruchas MR (2019) A motivational and neuropeptidergic hub: anatomical and functional diversity within nucleus accumbens shell. Neuron 102:529–552. https://doi.org/10.1016/j.neuron.2019.03.003
Cataldo G, Bernal S, Markowitz A, Ogawa S, Ragnauth A, Pfaff DW, Bodnar RJ (2005) Organizational manipulation of gonadal hormones and systemic morphine analgesia in female rats: effects of adult ovariectomy and estradiol replacement. Brain Res 1059:13–19
Cataldo G, Lovric J, Chen C-C, Pytte CL, Bodnar RJ (2010) Ventromedial and medial preoptic hypothalamic ibotenic acid lesions potentiate systemic morphine analgesia in female, but not male rats. Behav Brain Res 214:301–316
Chance WT (1980) Autoanalgesia: opiate and non-opiate mechanisms. Neurosci Biobehav Rev 4:55–67. https://doi.org/10.1016/0149-7634(80)90025-1
Chen Y, Mestek A, Liu J, Hurley JA, Yu L (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol 44:8–12
Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L (1994) Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett 347:279–283
Chen T et al (2016) Mechanism underlying the analgesic effect exerted by endomorphin-1 in the rat ventrolateral periaqueductal gray. Mol Neurobiol 53:2036–2053. https://doi.org/10.1007/s12035-015-9159-5
Cicero TJ, Nock B, Meyer ER (1996) Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther 279:767–773
Cicero TJ, Nock B, Meyer ER (1997) Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther 282:939–944
Cicero TJ, Nock B, O’Connor L, Meyer ER (2002) Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther 300:695–701
Clark FM, Proudfit HK (1991a) Projections of neurons in the ventromedial medulla to pontine catecholamine cell groups involved in the modulation of nociception. Brain Res 540:105–115
Clark FM, Proudfit HK (1991b) The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res 538:231–245
Clark FM, Proudfit HK (1991c) The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res 547:279–288
Clark FM, Proudfit HK (1993) The projections of noradrenergic neurons in the A5 catecholamine cell group to the spinal cord in the rat: anatomical evidence that A5 neurons modulate nociception. Brain Res 616:200–210
Clark JA, Itzhak Y, Hruby VJ, Yamamura HI, Pasternak GW (1986) [D-Pen2, D-Pen5]enkephalin (DPDPE): a δ-selective enkephalin with low affinity for μ1 opiate binding sites. Eur J Pharmacol 128:303–304
Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW (1989) Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther 251:461–468
Clements JR, Madl JE, Johnson RL, Larson AA, Beitz AJ (1987) Localization of glutamate, glutaminase, aspartate and aspartate aminotransferase in the rat midbrain periaqueductal gray. Exp Brain Res 67:594–602. https://doi.org/10.1007/BF00247290
Cortés R, Palacios JM (1986) Muscarinic cholinergic receptor subtypes in the rat brain. I. Quantitative autoradiographic studies. Brain Res 362:227–238. https://doi.org/10.1016/0006-8993(86)90448-8
Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ (1999) Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology 143:1–7
Czlonkowski A, Millan MJ, Herz A (1987) The selective κ-opioid agonist, U-50,488H, produces antinociception in the rat via a supraspinal action. Eur J Pharmacol 142:183–184
Dever SM, Costin BN, Xu R, El-Hage N, Balinang J, Samoshkin A, O’Brien MA, McRae M, Diatchenko L, Knapp PE, Hauser KF (2014) Differential expression of the alternatively spliced OPRM1 isoform μ-opioid receptor-1K in HIV-infected individuals. AIDS 28(1):19–30
Dickenson AH, Oliveras J-L, Besson J-M (1979) Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res 170:95–111
DiFeliceantonio AG, Berridge KC (2012) Which cue to ‘want’? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res 230:399–408. https://doi.org/10.1016/j.bbr.2012.02.032
Doyle HH, Murphy AZ (2018) Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiol Behav 187:32–41. https://doi.org/10.1016/j.physbeh.2017.11.030
Drugan RC, Grau JW, Maier SF, Madden J, Barchas JD (1981) Cross tolerance between morphine and the long-term analgesic reaction to inescapable shock. Pharmacol Biochem Behav 14:677–682
Ennis M, Behbehani M, Shipley MT, van Bockstaele EJ, Aston-Jones G (1991) Projections from the periaqueductal gray to the rostromedial pericoerulear region and nucleus locus coeruleus: anatomic and physiologic studies. J Comp Neurol 306:480–494
Eppler CM et al (1993) Purification and partial amino acid sequence of a mu opioid receptor from rat brain. J Biol Chem 268:26447–26451
Evans C, Keith D, Morrison H, Magendzo K, Edwards R (1992) Cloning of a delta opioid receptor by functional expression. Science 258:1952–1955
Fang FG, Fields HL, Lee NM (1986) Action at the mu receptor is sufficient to explain the supraspinal analgesic effect of opiates. J Pharmacol Exp Ther 238:1039–1044
Fields HL, Basbaum AI (1978) Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol 40:217–248
Fields HL, Margolis EB (2015) Understanding opioid reward. Trends Neurosci 38:217–225. https://doi.org/10.1016/j.tins.2015.01.002
Fields HL, Vanegas H, Hentall ID, Zorman G (1983) Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature 306:684–686
Fields HL, Heinricher MM, Mason P (1991) Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 14:219–245
Finley JCW, Maderdrut JL, Petrusz P (1981) The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol 198:541–565
Fowler C, Fraser G (1994) μ-, δ-, κ-Opioid receptors and their subtypes. A critical review with emphasis on radioligand binding experiments. Neurochem Int 24:401–426
Fukuda K, Kato S, Mori K, Nishi M, Takeshima H (1993) Primary structures and expression from cDNAs of rat opioid receptor δ-and μ-subtypes. FEBS Lett 327:311–314
Gallager DW, Pert A (1978) Afferents to brain stem nuclei (brain stem raphe, nucleus reticularis pontis caudalis and nucleus gigantocellularis) in the rat as demonstrated by microiontophoretically applied horseradish peroxidase. Brain Res 144:257–275
Galligan JJ, Mosberg HI, Hurst R, Hruby VJ, Burks TF (1984) Cerebral delta opioid receptors mediate analgesia but not the intestinal motility effects of intracerebroventricularly administered opioids. J Pharmacol Exp Ther 229:641–648
Gao K, Mason P (1997) Somatodendritic and axonal anatomy of intracellularly labeled serotonergic neurons in the rat medulla. J Comp Neurol 389:309–328
Gao K, Kim YH, Mason P (1997) SEROTONERGIC pontomedullary neurons are not activated by antinociceptive stimulation in the periaqueductal gray. J Neurosci 17:3285–3292
Gao K, Chen DO, Genzen JR, Mason P (1998) Activation of serotonergic neurons in the raphe magnus is not necessary for morphine analgesia. J Neurosci 18:1860–1868
Gebhart GF (1982) Opiate and opioid peptide effects on brain stem neurons: relevance to nociception and antinociceptive mechanisms. Pain 12:93–140
Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649
Girardot M, Holloway F (1984a) Cold water stress analgesia in rats: differential effects of naltrexone. Physiol Behav 32:547–555
Girardot M-N, Holloway FA (1984b) Intermittent cold water stress-analgesia in rats: cross-tolerance to morphine. Pharmacol Biochem Behav 20:631–633
Gistrak MA, Paul D, Hahn EF, Pasternak GW (1989) Pharmacological actions of a novel mixed opiate agonist/antagonist: naloxone benzoylhydrazone. J Pharmacol Exp Ther 251:469–476
Goldberg IE et al (1998) Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain. J Pharmacol Exp Ther 286:1007–1013
Goodman RR, Pasternak GW (1985) Visualization of mu1 opiate receptors in rat brain by using a computerized autoradiographic subtraction technique. Proc Natl Acad Sci 82:6667–6671
Goodman RR, Snyder SH, Kuhar MJ, Young WS (1980) Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci 77:6239–6243
Grau J, Hyson R, Maier S, Madden J, Barchas J (1981) Long-term stress-induced analgesia and activation of the opiate system. Science 213:1409–1411
Green PG, Kitchen I (1986) Antinociception opioids and the cholinergic system. Prog Neurobiol 26:119–146
Grinnell SG et al (2016) Mediation of buprenorphine analgesia by a combination of traditional and truncated mu opioid receptor splice variants. Synapse 70:395–407
Grisel JE, Mogil JS, Belknap JK, Grandy DK (1996) Orphanin FQ acts as a supraspinal, but not a spinal, anti-opioid peptide. NeuroReport 7:2125–2129
Guillemin R et al (1977) The endorphins, novel peptides of brain and hypophysial origin, with opiate-like activity: biochemical and biologic studies. Ann N Y Acad Sci 297:131–157
Hadjimarkou MM, Silva RM, Rossi GC, Pasternak GW, Bodnar RJ (2002) Feeding induced by food deprivation is differentially reduced by G-protein α-subunit antisense probes in rats. Brain Res 955:45–54
Hadjimarkou MM, Khaimova E, Pan Y-X, Rossi GC, Pasternak GW, Bodnar RJ (2003) Feeding induced by food deprivation is differentially reduced by opioid receptor antisense oligodeoxynucleotide probes in rats. Brain Res 987:223–232
Hadjimarkou MM et al (2004) Opioid receptor involvement in food deprivation-induced feeding: evaluation of selective antagonist and antisense oligodeoxynucleotide probe effects in mice and rats. J Pharmacol Exp Ther 311:1188–1202
Hadjimarkou MM, Abbadie C, Kasselman LJ, Pan Y-x, Pasternak GW, Bodnar RJ (2009) Changes in mouse mu opioid receptor Exon 7/8-like immunoreactivity following food restriction and food deprivation in rats. Synapse 63:585–597
Hammond DL, Yaksh TL (1984) Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res 298:329–337
Han W, Kasai S, Hata H, Takahashi T, Takamatsu Y, Yamamoto H, Uhl GR, Sora I, Ikeda K (2006) Intracisternal A-particle element in the 3′ noncoding region of the mu-opioid receptor gene in CXBK mice: a new genetic mechanism underlying differences in opioid sensitivity. Pharmacogenet Genom 16(6):451–460. https://doi.org/10.1097/01.fpc.0000215072.36965.8d
Harris HN, Peng YB (2020) Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural Regen Res 15:597–605. https://doi.org/10.4103/1673-5374.266909
Hasegawa Y, Kurachi M, Okuyama S, Araki H, Otomo S (1990) 5-HT3 receptor antagonists inhibit the response of κ oploid receptors in the morphine-reduced straub tail European. J Pharmacol 190:399–401
Hazum E, Chang K-J, Cuatrecasas P, Pasternak GW (1981) Naloxazone irreversibly inhibits the high affinity binding of [125I]D-ala2-D-leu5-enkephalin. Life Sci 28:2973–2979
Heinricher MM, Tortorici V (1994) Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience 63:533–546
Heinricher MM, Haws CM, Fields HL (1991) Evidence for GABA-mediated control of putative nociceptive modulating neurons in the rostral ventromedial medulla: iontophoresis of bicuculline eliminates the off-cell pause. Somatosens Mot Res 8:215–225
Heinricher MM, Morgan MM, Tortorici V, Fields HL (1994) Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience 63:279–288
Helmstetter FJ, Bellgowan PS, Tershner SA (1993) Inhibition of the tail flick reflex following microinjection of morphine into the amygdala. NeuroReport 4:471–474
Helmstetter FJ, Bellgowan PS, Poore LH (1995) Microinfusion of mu but not delta or kappa opioid agonists into the basolateral amygdala results in inhibition of the tail flick reflex in pentobarbital-anesthetized rats. J Pharmacol Exp Ther 275:381–388
Henderson G, McKnight AT (1997) The orphan opioid receptor and its endogenous ligand–nociceptin/orphanin FQ. Trends Pharmacol Sci 18:293–300
Herz A, Albus K, Metys̆ J, Schubert P, Teschemacher H (1970) On the central sites for the antinociceptive action of morphine and fentanyl. Neuropharmacology 9:539–551
Heyman JS, Mulvaney SA, Mosberg HI, Porreca F (1987) Opioid δ-receptor involvement in supraspinal and spinal antinociception in mice. Brain Res 420:100–108
Heyman JS, Williams CL, Burks TF, Mosberg HI, Porreca F (1988) Dissociation of opioid antinociception and central gastrointestinal propulsion in the mouse: studies with naloxonazine. J Pharmacol Exp Ther 245:238–243
Ho BY, Takemori AE (1990) Attenuation of the antinociceptive action of the selective κ-opioid receptor agonist, U-50,488H by ICS-205-930. Eur J Pharmacol 178:371–373
Hoehn K, Reid A, Sawynok J (1988) Pertussis toxin inhibits antinociception produced by intrathecal injection of morphine, noradrenaline and baclofen. Eur J Pharmacol 146:65–72
Hökfelt T, Elde R, Johansson O, Terenius L, Stein L (1977) The distribution of enkephalin-immunoreactive cell bodies in the rat central nervous system. Neurosci Lett 5:25–31
Holtzman SG (1974) Behavioral effects of separate and combined administration of naloxone and d-amphetamine. J Pharmacol Exp Ther 189:51–60
Hough LB, Nalwalk JW, Yang W, Ding X (2015) Neuronal cytochrome P450 activity and opioid analgesia: relevant sites and mechanisms. Brain Res 1616:10–18. https://doi.org/10.1016/j.brainres.2015.04.045
Huang YH, Wu YW, Chuang JY, Chang YC, Chang HF, Tao PL, Loh HH, Yeh SH (2020) Morphine produces potent antinociception, sedation, and hypothermia in humanized mice expressing human mu-opioid receptor splice variants. Pain 161(6):1177–1190. https://doi.org/10.1097/j.pain.0000000000001823.Pain
Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR (1975) Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 258:577–579
Hyson RL, Ashcraft LJ, Drugan RC, Grau JW, Maier SF (1982) Extent and control of shock affects naltrexone sensitivity of stress-induced analgesia and reactivity to morphine. Pharmacol Biochem Behav 17:1019–1025
Ikeda K, Kobayashi T, Ichikawa T, Kumanishi T, Niki H, Yano RJ (2001) The untranslated region of (mu)-opioid receptor mRNA contributes to reduced opioid sensitivity in CXBK mice. Neuroscience 21(4):1334–1339. https://doi.org/10.1523/JNEUROSCI.21-04-01334.2001
Ireson JD (1970) A comparison of the antinociceptive actions of cholinomimetic and morphine-like drugs. Br J Pharmacol 40:92–101
Islam AK, Cooper ML, Bodnar RJ (1993) Interactions among aging, gender, and gonadectomy effects upon morphine antinociception in rats. Physiol Behav 54:45–53
Israel Y et al (2005) NPY-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. Peptides 26:1167–1175
Itzhak Y, Pasternak GW (1987) Interaction of [D-Ser2, Leu5]enkephalin-Thr6 (DSLET), a relatively selective delta ligand, with MU1 opioid binding sites. Life Sci 40:307–311
Iwamoto ET (1989) Antinociception after nicotine administration into the mesopontine tegmentum of rats: evidence for muscarinic actions. J Pharmacol Exp Ther 251:412–421
Iwamoto ET (1991) Characterization of the antinociception induced by nicotine in the pedunculopontine tegmental nucleus and the nucleus raphe magnus. J Pharmacol Exp Ther 257:120–133
Jacquet YF (1988) The NMDA receptor: central role in pain inhibition in rat periaqueductal gray. Eur J Pharmacol 154:271–276
Jacquet YF, Lajtha A (1974) Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science 185:1055–1057. https://doi.org/10.1126/science.185.4156.1055
Jacquet YF, Lajtha A (1975) Morphine analgesia: 2-way cross tolerance between systematic and intracerebral (periaqueductal gray) administrations. Life Sci 17:1321–1324
Jacquet YF, Lajtha A (1976) The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res 103:501–513
Jensen TS, Yaksh TL (1984) Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res 321:287–297. https://doi.org/10.1016/0006-8993(84)90181-1
Jensen TS, Yaksh TL (1986a) III. Comparison of the antinociceptive action of Mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rat as studied by the microinjection technique. Brain Res 372:301–312
Jensen TS, Yaksh TL (1986b) II. Examination of spinal monoamine receptors through which brainstem opiate-sensitive systems act in the rat. Brain Res 363:114–127
Jensen TS, Yaksh TL (1986c) I. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Res 363:99–113
Jiang Q, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI, Porreca F (1991) Differential antagonism of opioid delta antinociception by [D-Ala2, Leu5, Cys6]enkephalin and naltrindole 5′-isothiocyanate: evidence for delta receptor subtypes. J Pharmacol Exp Ther 257:1069–1075
Kavaliers M, Innes DG (1990) Developmental changes in opiate-induced analgesia in deer mice: sex and population differences. Brain Res 516:326–331. https://doi.org/10.1016/0006-8993(90)90936-6
Keith DE Jr, Anton B, Evans CJ (1993) Characterization and mapping of a delta opioid receptor clone from NG108-15 cells. Proc West Pharmacol Soc 36:299–306
Kelly DD, Silverman A-J, Glusman M, Bodnar RJ (1993) Characterization of pituitary mediation of stress-induced antinociception in rats. Physiol Behav 53:769–775
Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ (1989) Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav 34:119–127
Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ (1991) Gender effects and central opioid analgesia. Pain 45:87–94
Kest B, Wilson SG, Mogil JS (1999) Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther 289:1370–1375
Khachaturian H, Watson SJ, Lewis ME, Coy D, Goldstein A, Akil H (1982) Dynorphin immunocytochemistry in the rat central nervous system. Peptides 3:941–954
Khachaturian H, Lewis M, Hollt V, Watson S (1983) Telencephalic enkephalinergic systems in the rat brain. J Neurosci 3:844–855
Khalilzadeh E, Saiah GV (2017) The possible mechanisms of analgesia produced by microinjection of morphine into the lateral habenula in the acute model of trigeminal pain in rats. Res Pharm Sci 12:241–248. https://doi.org/10.4103/1735-5362.207205
Kiefel JM, Cooper ML, Bodnar RJ (1992a) Serotonin receptor subtype antagonists in the medial ventral medulla inhibit mesencephalic opiate analgesia. Brain Res 597:331–338
Kiefel JM, Cooper ML, Bodnar RJ (1992b) Inhibition of mesencephalic morphine analgesia by methysergide in the medial ventral medulla of rats. Physiol Behav 51:201–205
Kiefel JM, Rossi GC, Bodnar RJ (1993) Medullary μ and δ opioid receptors modulate mesencephalic morphine analgesia in rats. Brain Res 624:151–161
Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG (1992) The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci 89:12048–12052
King MA, Rossi GC, Chang AH, Williams L, Pasternak GW (1997) Spinal analgesic activity of orphanin FQ/nociceptin and its fragments. Neurosci Lett 223:113–116
Kirchgessner AL, Bodnar RJ, Pasternak GW (1982) Naloxazone and pain-inhibitory systems: evidence for a collateral inhibition model. Pharmacol Biochem Behav 17:1175–1179
Kitanaka N, Sora I, Kinsey S, Zeng Z, Uhl GR (1998) No heroin or morphine 6β-glucuronide analgesia in μ-opioid receptor knockout mice. Eur J Pharmacol 355:R1–R3
Klamt JG, Prado WA (1991) Antinociception and behavioral changes induced by carbachol microinjected into identified sites of the rat brain. Brain Res 549:9–18
Klatt DS, Guinan MJ, Culhane ES, Carstens E, Watkins LR (1988) The dorsal raphe nucleus: a re-evaluation of its proposed role in opiate analgesia systems. Brain Res 447:246–252
Klein G, Rossi GC, Waxman AR, Arout C, Juni A, Inturrisi CE, Kest B (2009) The contribution of MOR-1 exons 1–4 to morphine and heroin analgesia and dependence. Neurosci Lett 457:115–119
Knapp RJ et al (1994) Identification of a human delta opioid receptor: cloning and expression. Life Sci 54:PL463–PL469
Knapp RJ et al (1995) Molecular biology and pharmacology of cloned opioid receptors sup1/sup. FASEB J 9:516–525
Krettek JE, Price JL (1978) Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 178:225–253
Kringel D, Lötsch J, Kringel D et al (2016) Next-generation sequencing of human opioid receptor genes based on a custom AmpliSeq™ library and ion torrent personal genome machine. Clin Chim Acta 463:32–38. https://doi.org/10.1016/j.cca.2016.10.009
Krzanowska EK, Bodnar RJ (1999) Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res 821:224–230
Krzanowska EK, Bodnar RJ (2000) Analysis of sex and gonadectomy differences in β-endorphin antinociception elicited from the ventrolateral periaqueductal gray in rats. Eur J Pharmacol 392:157–161
Krzanowska EK, Rossi GC, Pasternak GW, Bodnar RJ (1998) Potency ratios of morphine and morphine-6β-glucuronide analgesia elicited from the periaqueductal gray, locus coeruleus or rostral ventromedial medulla of rats. Brain Res 799:329–333
Krzanowska EK, Znamensky V, Wilk S, Bodnar RJ (2000) Antinociceptive and behavioral activation responses elicited by d-Pro(2)-endomorphin-2 in the ventrolateral periaqueductal gray are sensitive to sex and gonadectomy differences in rats. Peptides 21:705–715. https://doi.org/10.1016/s0196-9781(00)00191-1
Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ (2002) Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res 929:1–9
Kuraishi Y, Fukui K, Shiomi H, Akaike A, Takagi H (1978) Microinjection of opioids into the nucleus reticularis gigantocellularis of the rat: analgesia and increase in the normetanephrine level in the spinal cord. Biochem Pharmacol 27:2756–2758. https://doi.org/10.1016/0006-2952(78)90054-0
Lai J, Bilsky EJ, Rothman RB, Porreca F (1994) Treatment with antisense oligodeoxynucleotide to the opioid δ receptor selectively inhibits δ2-agonist antinociception. NeuroReport 5:1049–1052
Lauber AH, Romano GJ, Mobbs CV, Howells RD, Pfaff DW (1990) Estradiol induction of proenkephalin messenger RNA in hypothalamus: dose-response and relation to reproductive behavior in the female rat. Mol Brain Res 8:47–54
Letchworth SR, Mathis JP, Rossi GC, Bodnar RJ, Pasternak GW (2000) Autoradiographic localization of (125)I[Tyr(14)]orphanin FQ/nociceptin and (125)I[Tyr(10)]orphanin FQ/nociceptin(1-11) binding sites in rat brain. J Comp Neurol 423:319–329
Leventhal L, Cole JL, Rossi GC, Pan YX, Pasternak GW, Bodnar RJ (1996) Antisense oligodeoxynucleotides against the MOR-1 clone alter weight and ingestive responses in rats. Brain Res 719:78–84
Leventhal L, Stevens LB, Rossi GC, Pasternak GW, Bodnar RJ (1997) Antisense mapping of the MOR-1 opioid receptor clone: modulation of hyperphagia induced by DAMGO. J Pharmacol Exp Ther 282:1402–1407
Leventhal L, Mathis JP, Rossi GC, Pasternak GW, Bodnar RJ (1998a) Orphan opioid receptor antisense probes block orphanin FQ-induced hyperphagia. Eur J Pharmacol 349:R1–R3
Leventhal L, Silva RM, Rossi GC, Pasternak GW, Bodnar RJ (1998b) Morphine-6beta-glucuronide-induced hyperphagia: characterization of opioid action by selective antagonists and antisense mapping in rats. J Pharmacol Exp Ther 287:538–544
Levy RA, Proudfit HK (1979) Analgesia produced by microinjection of baclofen and morphine at brain stem sites. Eur J Pharmacol 57:43–55
Lewis J, Cannon J, Liebeskind J (1980) Opioid and nonopioid mechanisms of stress analgesia. Science 208:623–625
Lewis J, Sherman J, Liebeskind J (1981) Opioid and non-opioid stress analgesia: assessment of tolerance and cross-tolerance with morphine. J Neurosci 1:358–363
Lewis J, Tordoff M, Sherman J, Liebeskind J (1982) Adrenal medullary enkephalin-like peptides may mediate opioid stress analgesia. Science 217:557–559
Lewis ME, Khachaturian H, Watson SJ (1985) Combined autoradiographic-immunocytochemical analysis of opioid receptors and opioid peptide neuronal systems in brain. Peptides 6:37–47
Ling GS, Pasternak GW (1983) Spinal and supraspinal opioid analgesia in the mouse: the role of subpopulations of opioid binding sites. Brain Res 271:152–156. https://doi.org/10.1016/0006-8993(83)91376-8
Ling GSF, Spiegel K, Nishimura SL, Pasternak GW (1983) Dissociation of morphine’s analgesic and respiratory depressant actions. Eur J Pharmacol 86:487–488
Ling G, MacLeod J, Lee S, Lockhart S, Pasternak G (1984) Separation of morphine analgesia from physical dependence. Science 226:462–464
Ling GS, Spiegel K, Lockhart SH, Pasternak GW (1985) Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J Pharmacol Exp Ther 232:149–155
Ling GSF, Simantov R, Clark JA, Pasternak GW (1986) Naloxonazine actions in vivo. Eur J Pharmacol 129:33–38
Lipman JJ, Spencer PSJ (1980) A comparison of muscarinic cholinergic involvement in the antinociceptive effects of morphine and clonidine in the mouse. Eur J Pharmacol 64:249–258
Llewelyn M (1986) Brainstem mechanisms of antinociception Effects of electrical stimulation and injection of morphine into the nucleus raphe magnus. Neuropharmacology 25:727–735
Llewelyn MB, Azami J, Gibbs M, Roberts MHT (1983a) A comparison of the sites at which pentazocine and morphine act to produce analgesia. Pain 16:313–331
Llewelyn MB, Azami J, Roberts MHT (1983b) Effects of 5-hydroxytryptamine applied into nucleus raphe magnus on nociceptive thresholds and neuronal firing rate. Brain Res 258:59–68
Llorca-Torralba M, Mico JA, Berrocoso E (2018) Behavioral effects of combined morphine and MK-801 administration to the locus coeruleus of a rat neuropathic pain model. Prog Neuropsychopharmacol Biol Psychiatry 84:257–266. https://doi.org/10.1016/j.pnpbp.2018.03.007
Loh HH, Liu H-C, Cavalli A, Yang W, Chen Y-F, Wei L-N (1998) μ Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Mol Brain Res 54:321–326
London ED, Waller SB, Wamsley JK (1985) Autoradiographic localization of [3H]nicotine binding sites in the rat brain. Neurosci Lett 53:179–184
Loyd DR, Murphy AZ (2006) Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol 496:723–738
Loyd DR, Murphy AZ (2008) Androgen and estrogen (alpha) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. J Chem Neuroanat 36:216–226. https://doi.org/10.1016/j.jchemneu.2008.08.001
Loyd DR, Morgan MM, Murphy AZ (2007) Morphine preferentially activates the periaqueductal gray–rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience 147:456–468
Loyd DR, Morgan MM, Murphy AZ (2008a) Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci 27:1517–1524. https://doi.org/10.1111/j.1460-9568.2008.06100.x
Loyd DR, Wang X, Murphy AZ (2008b) Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci 28:14007–14017. https://doi.org/10.1523/JNEUROSCI.4123-08.2008
Lu Z, Xu J, Rossi GC, Majumdar S, Pasternak GW, Pan Y-X (2015) Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Investig 125:2626–2630
Lu Z, Xu J, Xu M, Rossi GC, Majumdar S, Pasternak GW, Pan YX (2018) Truncated μ-opioid receptors with 6 transmembrane domains are essential for opioid analgesia. Anesth Analg 126:1050–1057. https://doi.org/10.1213/ANE.0000000000002538
Ma QP, Shi YS, Han JS (1992) Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res 583:292–295. https://doi.org/10.1016/s0006-8993(10)80036-8
MacLennan A, Drugan R, Hyson R, Maier S, Madden J, Barchas J (1982a) Corticosterone: a critical factor in an opioid form of stress-induced analgesia. Science 215:1530–1532
MacLennan AJ, Drugan RC, Hyson RL, Maier SF, Madden J, Barchas JD (1982b) Dissociation of long-term analgesia and the shuttle box escape deficit caused by inescapable shock. J Comp Physiol Psychol 96:904–912
Majumdar S et al (2011) Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci 108:19778–19783
Majumdar S, Subrath J, Le Rouzic V, Polikar L, Burgman M, Nagakura K, Ocampo J, Haselton N, Pasternak AR, Grinnell S, Pan YX, Pasternak GW (2012) Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated μ opioid receptor (MOR-1) splice variants. J Med Chem 55(14):6352–6362. https://doi.org/10.1021/jm300305c
Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1987) Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7:2445–2464
Mansour A, Fox CA, Akil H, Watson SJ (1995a) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18:22–29. https://doi.org/10.1016/0166-2236(95)93946-u
Mansour A, Fox CA, Burke S, Akil H, Watson SJ (1995b) Immunohistochemical localization of the cloned μ opioid receptor in the rat CNS. J Chem Neuroanat 8:283–305
Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ (1996) Immunohistochemical localization of the cloned κ1 receptor in the rat CNS and pituitary. Neuroscience 71:671–690
Marek P, Panocka I, Hartmann G (1982) Enhancement of stress-induced analgesia in adrenalectomized mice: its reversal by dexamethasone. Pharmacol Biochem Behav 16:403–405
Marek P, Panocka I, Sadowski B (1983) Dexamethasone reverses adrenalectomy enhancement of footshock induced analgesia in mice. Pharmacol Biochem Behav 18:167–169
Marek P, Panocka I, Sadowski B (1986) Activation of anti- and pro-nociceptive mechanisms by front paw shock in spinal mice: involvement of humoral factors. Pharmacol Biochem Behav 24:791–793
Marrone GF et al (2016a) Truncated mu opioid GPCR variant involvement in opioid-dependent and opioid-independent pain modulatory systems within the CNS. Proc Natl Acad Sci 113:3663–3668
Marrone GF et al (2016b) Tetrapeptide endomorphin analogs require both full length and truncated splice variants of the mu opioid receptor gene Oprm1 for analgesia. ACS Chem Neurosci 7:1717–1727. https://doi.org/10.1021/acschemneuro.6b00240
Marrone GF et al (2017) Genetic dissociation of morphine analgesia from hyperalgesia in mice. Psychopharmacology 234:1891–1900. https://doi.org/10.1007/s00213-017-4600-2
Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE (1976) The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197:517–532
Mason P (1997) Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol 77:1087–1098
Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RW IV, McCall JG, Al-Hasani R, Bruchas MR, Morón JA (2019) Pain-Induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102:564–573. https://doi.org/10.1016/j.neuron.2019.02.029
Mathis JP, Goldberg IE, Rossi GC, Leventhal L, Pasternak GW (1998) Antinociceptive analogs of orphanin FQ/nociceptin(1-11). Life Sci 63:PL161–PL166. https://doi.org/10.1016/s0024-3205(98)00358-0
Mathis JP, Rossi GC, Pellegrino MJ, Jimenez C, Pasternak GW, Allen RG (2001) Carboxyl terminal peptides derived from prepro-orphanin FQ/nociceptin (ppOFQ/N) are produced in the hypothalamus and possess analgesic bioactivities. Brain Res 895:89–94. https://doi.org/10.1016/s0006-8993(01)02035-2
Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioidreceptorgene. Nature 383(6603):819–823. https://doi.org/10.1038/383819a0
Mattia A, Vanderah T, Mosberg HI, Porreca F (1991) Lack of antinociceptive cross-tolerance between [D-Pen2, D-Pen5]enkephalin and [D-Ala2]deltorphin II in mice: evidence for delta receptor subtypes. J Pharmacol Exp Ther 258:583–587
Mattia A et al (1992) Spinal opioid delta antinociception in the mouse: mediation by a 5′-NTII-sensitive delta receptor subtype. J Pharmacol Exp Ther 260:518–525
Mayer DJ, Price DD (1976) Central nervous system mechanisms of analgesia. Pain 2:379–404
McGowan MK, Hammond DL (1993a) Intrathecal GABAB antagonists attenuate the antinociception produced by microinjection ofl-glutamate into the ventromedial medulla of the rat. Brain Res 607:39–46
McGowan MK, Hammond DL (1993b) Antinociception produced by microinjection ofl-glutamate into the ventromedial medulla of the rat: mediation by spinal GABAA receptors. Brain Res 620:86–96
Meunier J-C et al (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535
Millan MJ, Gramsch C, Przewłocki R, Höllt V, Herz A (1980a) Lesions of the hypothalamic arcuate nucleus produce a temporary hyperalgesia and attenuate stress-evoked analgesia. Life Sci 27:1513–1523
Millan MJ, Przewlocki R, Herz A (1980b) A non-beta-endorphinergic adenohypophyseal mechanism is essential for an analgetic response to stress. Pain 8:343–353
Min BH, Augustin LB, Felsheim RF, Fuchs JA, Loh HH (1994) Genomic structure analysis of promoter sequence of a mouse mu opioid receptor gene. Proc Natl Acad Sci USA 91:9081–9085. https://doi.org/10.1073/pnas.91.19.9081
Minami M et al (1993) Cloning and expression of a cDNA for the rat ik/i-opioid receptor. FEBS Lett 329:291–295
Mitchell JM, Lowe D, Fields HL (1998) The contribution of the rostral ventromedial medulla to the antinociceptive effects of systemic morphine in restrained and unrestrained rats. Neuroscience 87:123–133
Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC (1993) Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain 53:17–25
Mogil JSKB, Sadowski B, Belknap JK (1996a) Differential genetic mediation of sensitivity to morphine in genetic models of opiate antinociception: influence of nociceptive assay. J Pharmacol Exp Ther 276:532–544
Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK (1996b) Orphanin FQ is a functional anti-opioid peptide. Neuroscience 75:333–337. https://doi.org/10.1016/0306-4522(96)00338-7
Mollereau C et al (1994) ORL1, a novel member of the opioid receptor family: cloning, functional expression and localization. FEBS Lett 341:33–38
Monroe PJ, Hawranko AA, Smith DL, Smith DJ (1996) Biochemical and pharmacological characterization of multiple beta-endorphinergic antinociceptive systems in the rat periaqueductal gray. J Pharmacol Exp Ther 276:65–73
Morgan MM, Heinricher MM, Fields HL (1992) Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience 47:863–871
Morgan MM, Reid RA, Stormann TM, Lautermilch NJ (2014) Opioid selective antinociception following microinjection into the periaqueductal gray of the rat. J Pain 15:1102–1109. https://doi.org/10.1016/j.jpain.2014.07.008
Mosberg HI, Hurst R, Hruby VJ, Galligan JJ, Burks TF, Gee K, Yamamura HI (1983a) Conformationally constrained cyclic enkephalin analogs with pronounced delta opioid receptor agonist selectivity. Life Sci 32:2565–2569. https://doi.org/10.1016/0024-3205(83)90239-4
Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, Burks TF (1983b) Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci 80:5871–5874
Moskowitz AS, Goodman RR (1985a) Autoradiographic analysis of mu1, mu2, and delta opioid binding in the central nervous system of C57BL/6BY and CXBK (opioid receptor-deficient) mice. Brain Res 360:108–116
Moskowitz AS, Goodman RR (1985b) Autoradiographic distribution of Mu1 and Mu2 opioid binding in the mouse central nervous system. Brain Res 360:117–129
Mousa S, Miller CH Jr, Couri D (1981) Corticosteroid modulation and stress-induced analgesia in rats. Neuroendocrinology 33:317–319
Mousa S, Miller CH Jr, Couri D (1983) Dexamethasone and stress-induced analgesia. Psychopharmacology 79:199–202. https://doi.org/10.1007/bf00427812
Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F (2012) Pain relief produces negative reinforcement through activation of mesolimbic reward–valuation circuitry. Proc Natl Acad Sci USA 109(50):20709–20713. https://doi.org/10.1073/pnas.1214605109
Nishimura SL, Recht LD, Pasternak GW (1984) Biochemical characterization of high-affinity 3H-opioid binding. Further evidence for Mu1 sites. Mol Pharmacol 25:29–37
Pan YX (2002) Identification and characterization of a novel promoter of the mouse mu opioid receptor gene (Oprm) that generates eight splice variants. Gene 295:97–108. https://doi.org/10.1016/s0378-1119(02)00825-9
Pan ZZ, Fields HL (1996) Endogenous opioid-mediated inhibition of putative pain-modulating neurons in rat rostral ventromedial medulla. Neuroscience 74:855–862
Pan Y-X, Pasternak GW (2011) Molecular biology of mu opioid receptors. In: Pasternak GW (ed) The opiate receptors. Humana Press, Totowa, pp 121–160. https://doi.org/10.1007/978-1-60761-993-2_6
Pan YX et al (1995) Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol Pharmacol 47:1180–1188
Pan Y-X et al (1999) Identification and characterization of three new alternatively spliced μ-opioid receptor isoforms. Mol Pharmacol 56:396–403
Pan Y-X, Xu J, Bolan E, Chang A, Mahurter L, Rossi G, Pasternak GW (2000) Isolation and expression of a novel alternatively spliced mu opioid receptor isoform, MOR-1F. FEBS Lett 466:337–340
Pan Y-X, Xu J, Mahurter L, Bolan E, Xu M, Pasternak GW (2001) Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc Natl Acad Sci 98:14084–14089
Pan YX, Xu J, Mahurter L, Xu M, Gilbert AK, Pasternak GW (2003) Identification and characterization of two new human mu opioid receptor splice variants, hMOR-1O and hMOR-1X. Biochem Biophys Res Commun 301(4):1057–1061. https://doi.org/10.1016/s0006-291x(03)00089-5
Pan L, Xu J, Yu R, Xu MM, Pan YX, Pasternak GW (2005a) Identification and characterization of six new alternatively spliced variants of the human mu opioid receptor gene, Oprm. Neuroscience 133(1):209–220. https://doi.org/10.1016/j.neuroscience.2004.12.033
Pan Y-X, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW (2005b) Identification of four novel exon 5 splice variants of the mouse μ-opioid receptor gene: functional consequences of C-terminal splicing. Mol Pharmacol 68:866–875
Pan Y-X, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW (2009) Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci 106:4917–4922
Panocka I, Sadowski B, Marek P (1987) Adrenalectomy and dexamethasone differentially affect postswim antinociception in mice selectively bred for high and low stress-induced analgesia. Physiol Behav 40:597–601
Pare WP (1969) Age, sex, and strain differences in the aversive threshold to grid shock in the rat. J Comp Physiol Psychol 69:214–218
Parenti M, Tirone F, Giagnoni G, Pecora N, Parolaro D (1986) Pertussis toxin inhibits the antinociceptive action of morphine in the rat. Eur J Pharmacol 124:357–359
Pasternak GW (1980) Multiple opiate receptors: [3H]ethylketocyclazocine receptor binding and ketocyclazocine analgesia. Proc Natl Acad Sci 77:3691–3694
Pasternak GW, Pan Y-X (2013) Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65:1257–1317
Pasternak GW, Wood PJ (1986) Minireview: multiple MU opiate receptors. Life Sci 38:1889–1898
Pasternak G, Childers, Snyder S (1980a) Opiate analgesia: evidence for mediation by a subpopulation of opiate receptors. Science 208:514–516
Pasternak GW, Childers SR, Snyder SH (1980b) Naloxazone, a long-acting opiate antagonist: effects on analgesia in intact animals and on opiate receptor binding in vitro. J Pharmacol Exp Ther 214:455–462
Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE (1987) Morphine-6-glucuronide, a potent mu agonist. Life Sci 41:2845–2849. https://doi.org/10.1016/0024-3205(87)90431-0
Pasternak KR, Rossi GC, Zuckerman A, Pasternak GW (1999) Antisense mapping KOR-1: evidence for multiple kappa analgesic mechanisms. Brain Res 826:289–292
Paul D, Phillips AG (1986) Selective effects of pirenperone on analgesia produced by morphine or electrical stimulation at sites in the nucleus raphe magnus and periaqueductal gray. Psychopharmacology 88:172–176. https://doi.org/10.1007/bf00652235
Paul D, Mana MJ, Pfaus JG, Pinel JP (1988) Attenuation of morphine analgesia by the S2 antagonists, pirenperone and ketanserin. Pharmacol Biochem Behav 31:641–647. https://doi.org/10.1016/0091-3057(88)90243-2
Paul D, Bodnar RJ, Gistrak MA, Pasternak GW (1989a) Different mu receptor subtypes mediate spinal and supraspinal analgesia in mice. Eur J Pharmacol 168:307–314. https://doi.org/10.1016/0014-2999(89)90792-9
Paul D, Standifer KM, Inturrisi CE, Pasternak GW (1989b) Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J Pharmacol Exp Ther 251:477–483
Paul D, Levison JA, Howard DH, Pick CG, Hahn EF, Pasternak GW (1990) Naloxone benzoylhydrazone (NalBzoH) analgesia. J Pharmacol Exp Ther 255:769–774
Pavlovic ZW, Bodnar RJ (1998a) U50488H-induced analgesia in the amygdala: test-specific effects and blockade by opioid antagonists in the periaqueductal gray. Analgesia 3:223–230
Pavlovic ZW, Bodnar RJ (1998b) Opioid supraspinal analgesic synergy between the amygdala and periaqueductal gray in rats. Brain Res 779:158–169
Pavlovic ZW, Cooper ML, Bodnar RJ (1996) Opioid antagonists in the periaqueductal gray inhibit morphine and β-endorphin analgesia elicited from the amygdala of rats. Brain Res 741:13–26
Pazos A, Palacios JM (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res 346:205–230
Pazos A, Cortés R, Palacios JM (1985) Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res 346:231–249
Pert CB, Snyder SH (1973) Opiate receptor: demonstration in nervous tissue. Science 179:1011–1014
Pert A, Yaksh T (1974) Sites of morphine induced analgesia in the primate brain: relation to pain pathways. Brain Res 80:135–140. https://doi.org/10.1016/0006-8993(74)90731-8
Phoenix CH, Goy RW, Gerall AA, Young WC (1959) Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382. https://doi.org/10.1210/endo-65-3-369
Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF (1984) Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther 230:341–348
Porreca F, Heyman JS, Mosberg HI, Omnaas JR, Vaught JL (1987) Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. J Pharmacol Exp Ther 241:393–400
Potrebic S, Fields H, Mason P (1994) Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci 14:1655–1665
Potrebic S, Mason P, Fields H (1995) The density and distribution of serotonergic appositions onto identified neurons in the rat rostral ventromedial medulla. J Neurosci 15:3273–3283
Prieto GJ, Cannon JT, Liebeskind JC (1983) N. raphe magnus lesions disrupt stimulation-produced analgesia from ventral but not dorsal midbrain areas in the rat. Brain Res 261:53–57
Proudfit HK (1980) Reversible inactivation of raphe magnus neurons: effects on nociceptive threshold and morphine-induced analgesia. Brain Res 201:459–464
Proudfit HK, Hammond DL (1981) Alterations in nociceptive threshold and morphine-induced analgesia produced by intrathecally administered amine antagonists. Brain Res 218:393–399
Przewłocka B, Mika J, Łabuz D, Toth G, Przewłocki R (1999) Spinal analgesic action of endomorphins in acute, inflammatory and neuropathic pain in rats. Eur J Pharmacol 367:189–196
Przewłocki R, Costa T, Lang J, Herz A (1987) Pertussis toxin abolishes the antinociception mediated by opioid receptors in rat spinal cord. Eur J Pharmacol 144:91–95
Raffa RB, Martinez RP, Connelly CD (1994) G-protein antisense oligodeoxyribonucleotides and μ-opioid supraspinal antinociception. Eur J Pharmacol 258:R5–R7
Rainbow TC, Schwartz RD, Parsons B, Kellar KJ (1984) Quantitative autoradiography of nicotinic [3H]acetylcholine binding sites in rat brain. Neurosci Lett 50:193–196
Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T (1994) Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol 45:330–334
Reinscheid RK et al (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794
Reisine T, Bell GI (1993) Molecular biology of opioid receptors. Trends Neurosci 16:506–510
Reny-Palasse V, Monier C, Rips R (1989) Opioid involvement in TRH-induced antinociception in the rat following intracerebral administration. Pain 38:193–201. https://doi.org/10.1016/0304-3959(89)90238-8
Reynolds DV (1969) Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164:444–445
Rizvi TA, Ennis M, Behbehani MM, Shipley MT (1991) Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol 303:121–131
Robertson JA, Bodnar RJ (1993) Site-specific modulation of morphine and swim-induced antinociception following thyrotropin-releasing hormone in the rat periaqueductal gray. Pain 55:71–84
Rodgers RJ (1977) Elevation of aversive threshold in rats by intra-amygdaloid injection of morphine sulphate. Pharmacol Biochem Behav 6:385–390
Rodgers RJ (1978) Influence of intra-amygdaloid opiate injections on shock thresholds, tail-flick latencies and open field behaviour in rats. Brain Res 153:211–216
Roerig SC, Fujimoto JM (1989) Multiplicative interaction between intrathecally and intracerebroventricularly administered mu opioid agonists but limited interactions between delta and kappa agonists for antinociception in mice. J Pharmacol Exp Ther 249:762–768
Roerig SC, Fujimoto JM, Tseng LF (1988) Comparisons of descending pain inhibitory pathways activated by beta-endorphin and morphine as characterized by supraspinal and spinal antinociceptive interactions in mice. J Pharmacol Exp Ther 247:1107–1113
Romano GJ, Harlan RE, Shivers BD, Howells RD, Pfaff DW (1988) Estrogen increases proenkephalin messenger ribonucleic acid levels in the ventromedial hypothalamus of the rat. Mol Endocrinol 2:1320–1328
Romano GJ, Mobbs CV, Howells RD, Pfaff DW (1989) Estrogen regulation of proenkephalin gene expression in the ventromedial hypothalamus of the rat: temporal qualities and synergism with progesterone. Mol Brain Res 5:51–58
Romano GJ, Mobbs CV, Lauber A, Howells RD, Pfaff DW (1990) Differential regulation of proenkephalin gene expression by estrogen in the ventromedial hypothalamus of male and female rats: implications for the molecular basis of a sexually differentiated behavior. Brain Res 536:63–68
Romero M-T, Bodnar RJ (1986) Gender differences in two forms of cold-water swim analgesia. Physiol Behav 37:893–897
Romero M-T, Kepler KL, Cooper ML, Komisaruk BR, Bodnar RJ (1987) Modulation of gender-specific effects upon swim analgesia in gonadectomized rats. Physiol Behav 40:39–45
Romero M-T, Cooper ML, Komisaruk BR, Bodnar RJ (1988) Gender-specific and gonadectomy-specific effects upon swim analgesia: role of steroid replacement therapy. Physiol Behav 44:257–265
Root DH, Melendez RI, Zaborszky L, Napier TC (2015) The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130:29–70. https://doi.org/10.1016/j.pneurobio.2015.03.005
Rossi GC, Pasternak G (1997) Establishing the molecular biology of opioid behavior through antisense approaches. In: Weiss (ed) Antisense oligodeoxynucleotides and antisense RNA: novel pharmacological and therapeutics. CRC Press, New York, pp 115–130
Rossi GC, Pasternak GW, Bodnar RJ (1993) Synergistic brainstem interactions for morphine analgesia. Brain Res 624:171–180
Rossi G, Pan Y-X, Cheng J, Pasternak GW (1994a) Blockade of morphine analgesia by an antisense oligodeoxynucleotide against the mu receptor. Life Sciences 54:PL375–PL379
Rossi GC, Pasternak GW, Bodnar RJ (1994b) μ and δ opioid synergy between the periaqueductal gray and the rostro-ventral medulla. Brain Res 665:85–93
Rossi GC, Pan Y-X, Brown GP, Pasternak GW (1995a) Antisense mapping the MOR-1 opioid receptor: evidence for alternative splicing and a novel morphine-6β-glucuronide receptor. FEBS Lett 369:192–196
Rossi GC, Standifer KM, Pasternak GW (1995b) Differential blockade of morphine and morphine-6β-glucuronide analgesia by antisense oligodeoxynucleotides directed against MOR-1 and G-protein α subunits in rats. Neurosci Lett 198:99–102
Rossi GC, Brown GP, Leventhal L, Yang K, Pasternak GW (1996a) Novel receptor mechanisms for heroin and morphine-6β-glucuronide analgesia. Neurosci Lett 216:1–4
Rossi GC, Leventhal L, Pasternak GW (1996b) Naloxone sensitive orphanin FQ-induced analgesia in mice. Eur J Pharmacol 311:R7–R8
Rossi GC, Leventhal L, Bolan E, Pasternak GW (1997a) Pharmacological characterization of orphanin FQ/nociceptin and its fragments. J Pharmacol Exp Ther 282:858–865
Rossi GC, Leventhal L, Pan YX, Cole J, Su W, Bodnar RJ, Pasternak GW (1997b) Antisense mapping of MOR-1 in rats: distinguishing between morphine and morphine-6beta-glucuronide antinociception. J Pharmacol Exp Ther 281:109–114
Rossi GC, Su W, Leventhal L, Su H, Pasternak GW (1997c) Antisense mapping DOR-1 in mice: further support for δ receptor subtypes. Brain Res 753:176–179
Rossi GC, Mathis JP, Pasternak GW (1998a) Analgesic activity of orphanin FQ2, murine prepro-orphanin FQ141-157 in mice. NeuroReport 9:1165–1168
Rossi GC, Perlmutter M, Leventhal L, Talatti A, Pasternak GW (1998b) Orphanin FQ/nociceptin analgesia in the rat. Brain Res 792:327–330
Rossi GC et al (2002) Characterization of rat prepro-orphanin FQ/nociceptin((154-181)): nociceptive processing in supraspinal sites. J Pharmacol Exp Ther 300:257–264. https://doi.org/10.1124/jpet.300.1.257
Roychowdhury SM, Fields HL (1996) Endogenous opioids acting at a medullary μ-opioid receptor contribute to the behavioral antinociception produced by GABA antagonism in the midbrain periaqueductal gray. Neuroscience 74:863–872
Roychowdhury SM, Heinricher MM (1997) Effects of iontophoretically applied serotonin on three classes of physiologically characterized putative pain modulating neurons in the rostral ventromedial medulla of lightly anesthetized rats. Neurosci Lett 226:136–138
Ryan SM, Maier SF (1988) The estrous cycle and estrogen modulate stress-induced analgesia. Behav Neurosci 102:371–380
Rye DB, Lee HJ, Saper CB, Wainer BH (1988) Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 269:315–341
Sanchez-Blazquez P, Garcia-Espana A, Garzon J (1995) In vivo injection of antisense oligodeoxynucleotides to G alpha subunits and supraspinal analgesia evoked by mu and delta opioid agonists. J Pharmacol Exp Ther 275:1590–1596
Sandkühler J, Gebhart GF (1984) Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res 305:77–87
Sathaye N, Bodnar RJ (1989) Dissociation of opioid and nonopioid analgesic responses following adult monosodium glutamate pretreatment. Physiol Behav 46:217–222
Satoh M, Kubota A, Iwama T, Wada T, Yasui M, Fujibayashi K, Takagi H (1983a) Comparison of analgesic potencies of mu, delta and kappa agonists locally applied to various CNS regions relevant to analgesia in rats. Life Sci 33:689–692
Satoh M, Oku R, Akaike A (1983b) Analgesia produced by microinjection of-glutamate into the rostral ventromedial bulbar nuclei of the rat and its inhibition by intrathecal α-adrenergic blocking agents. Brain Res 261:361–364
Schmauss C, Yaksh TL (1984) In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther 228:1–12
Schmidt BL, Tambeli CH, Barletta J, Luo L, Green P, Levine JD, Gear RW (2002) Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. J Neurosci 22:6773–6780. https://doi.org/10.1523/JNEUROSCI.22-15-06773.2002
Schuller AG et al (1999) Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2:151–156. https://doi.org/10.1038/5706
Segal M, Sandberg D (1977) Analgesia produced by electrical stimulation of catecholamine nuclei in the rat brain. Brain Res 123:369–372
Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, Tchivileva IE, Belfer I, Mishra B, Kiselycznyk C, Wallace MR, Staud R, Spiridonov NA, Max MB, Goldman D, Fillingim RB, Maixner W, Diatchenko L (2009) Expansion of the human mu-opioid receptor gene architecture: novel functional variants. Hum Mol Genet 18(6):1037–1051
Shane R, Wilk S, Bodnar RJ (1999) Modulation of endomorphin-2-induced analgesia by dipeptidyl peptidase IV. Brain Res 815:278–286. https://doi.org/10.1016/s0006-8993(98)01121-4
Shane R, Lazar DA, Rossi GC, Pasternak GW, Bodnar RJ (2001) Analgesia elicited by OFQ/nociceptin and its fragments from the amygdala in rats. Brain Res 907:109–116
Shane R, Acosta J, Rossi GC, Bodnar RJ (2003) Reciprocal interactions between the amygdala and ventrolateral periaqueductal gray in mediating of Q/N(1-17)-induced analgesia in the rat. Brain Res 980:57–70. https://doi.org/10.1016/s0006-8993(03)02887-7
Sharpe LG, Garnett JE, Cicero TJ (1974) Analgesia and hyperreactivity produced by intracranial microinjections of morphine into the periaqueductal gray matter of the rat. Behav Biol 11:303–313
Shimomura K, Kamata O, Ueki S, Ida S, Oguri K, Yoshimura H, Tsukamoto H (1971) Analgesic effect of morphine glucuronides. Tohoku J Exp Med 105:45–52. https://doi.org/10.1620/tjem.105.45
Shiromani PJ, Armstrong DM, Gillin JC (1988) Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci Lett 95:19–23
Siegfried B, de Souza RL (1989) NMDA receptor blockade in the periaqueductal grey prevents stress-induced analgesia in attacked mice. Eur J Pharmacol 168:239–242. https://doi.org/10.1016/0014-2999(89)90570-0
Silva RM, Rossi GC, Mathis JP, Standifer KM, Pasternak GW, Bodnar RJ (2000) Morphine and morphine-6β-glucuronide-induced feeding are differentially reduced by G-protein α-subunit antisense probes in rats. Brain Res 876:62–75
Silva RM, Hadjimarkou MM, Rossi GC, Pasternak GW, Bodnar RJ (2001) Beta-endorphin-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. J Pharmacol Exp Ther 297:590–596
Silva RM, Grossman HC, Hadjimarkou MM, Rossi GC, Pasternak GW, Bodnar RJ (2002a) Dynorphin A(1-17)-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. J Pharmacol Exp Ther 301:513–518. https://doi.org/10.1124/jpet.301.2.513
Silva RM, Grossman HC, Rossi GC, Pasternak GW, Bodnar RJ (2002b) Pharmacological characterization of β-endorphin- and dynorphin A1–17-induced feeding using G-protein α-subunit antisense probes in rats. Peptides 23:1101–1106
Simon EJ, Hiller JM, Edelman I (1973) Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci USA 70:1947–1949. https://doi.org/10.1073/pnas.70.7.1947
Simone DA, Bodnar RJ, Goldman EJ, Pasternak GW (1985) Involvement of opioid receptor subtypes in rat feeding behavior. Life Sci 36:829–833
Skinner K, Fields HL, Basbaum AI, Mason P (1997) GABA-immunoreactive boutons contact identified OFF and ON cells in the nucleus raphe magnus. J Comp Neurol 378:196–204
Smith DJ, Perrotti JM, Crisp T, Cabral MEY, Long JT, Scalzitti JM (1988) The μ opiate receptor is responsible for descending pain inhibition originating in the periaqueductal gray region of the rat brain. Eur J Pharmacol 156:47–54
Smith D, Robertson B, Monroe P, Taylor D, Leedham J, Cabral J (1992a) Opioid receptors mediating antinociception from β-endorphin and morphine in the periaqueductal gray. Neuropharmacology 31:1137–1150
Smith DJ, Robertson B, Monroe PJ (1992b) Antinociception from the administration of β-endorphin into the periaqueductal gray of rat is enhanced while that of morphine is inhibited by barbiturate anesthesia. Neurosci Lett 146:143–146
Sora I et al (1997) Opiate receptor knockout mice define receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci 94:1544–1549
Spencer DG, Horváth E, Traber Jr (1986) Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res 380:59–68
Sperber ES, Romero M-T, Bodnar RJ (1986) Selective potentiations in opioid analgesia following scopolamine pretreatment. Psychopharmacology 89:175–176
Spiegel K, Kourides I, Pasternak G (1982) Prolactin and growth hormone release by morphine in the rat: different receptor mechanisms. Science 217:745–747
Spinella M, Cooper ML, Bodnar RJ (1996) Excitatory amino acid antagonists in the rostral ventromedial medulla inhibit mesencephalic morphine analgesia in rats. Pain 64:545–552
Spinella M, Znamensky V, Moroz M, Ragnauth A, Bodnar RJ (1999) Actions of NMDA and cholinergic receptor antagonists in the rostral ventromedial medulla upon β-endorphin analgesia elicited from the ventrolateral periaqueductal gray. Brain Res 829:151–159
Sprenger C, Eichler IC, Eichler L, Zöllner C, Büchel C (2018) Altered signaling in the descending pain-modulatory system after short-term infusion of the μ-opioid agonist remifentanil. J Neurosci 38:2454–2470. https://doi.org/10.1523/JNEUROSCI.2496-17.2018
Standifer KM, Chien C-C, Wahlested C, Brown GP, Pasternak GW (1994) Selective loss of δ opioid analgesia and binding by antisense oligodeoxynucleotides to a δ opioid receptor. Neuron 12:805–810
Standifer KM, Rossi GC, Pasternak GW (1996) Differential blockade of opioid analgesia by antisense oligodeoxynucleotides directed against various G protein alpha subunits. Mol Pharmacol 50:293–298
Stein JA, Znamensky V, Baumer F, Rossi GC, Pasternak GW, Bodnar RJ (2000) Mercaptoacetate induces feeding through central opioid-mediated mechanisms in rats. Brain Res 864:240–251
Steinman JL, Faris PL, Mann PE, Olney JW, Komisaruk BR, Willis WD, Bodnar RJ (1990) Antagonism of morphine analgesia by nonopioid cold-water swim analgesia: direct evidence for collateral inhibition. Neurosci Biobehav Rev 14:1–7. https://doi.org/10.1016/s0149-7634(05)80155-1
Stoffel EC, Ulibarri CM, Craft RM (2003) Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain 103:285–302
Stoffel E, Ulibarri C, Folk J, Rice K, Craft R (2005) Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain 6:261–274
Stone LS, Vulchanova L (2003) The pain of antisense: in vivo application of antisense oligonucleotides for functional genomics in pain and analgesia. Adv Drug Deliv Rev 55(8):1081–1112. https://doi.org/10.1016/s0169-409x(03)00105-4
Stone LS, Fairbanks CA, Laughlin TM, Nguyen HO, Bushy TM, Wessendorf MW, Wilcox GL (1997) Spinal analgesic actions of the new endogenous opioid peptides endomorphin-1 and -2. NeuroReport 8:3131–3135. https://doi.org/10.1097/00001756-199709290-00025
Suh HH, Tseng LF (1990a) Lack of antinociceptive cross-tolerance between intracerebroventricularly administered β-endorphin and morphine or DPDPE in mice. Life Sci 46:759–765
Suh HH, Tseng LF (1990b) Delta but not mu-opioid receptors in the spinal cord are involved in antinociception induced by beta-endorphin given intracerebroventricularly in mice. J Pharmacol Exp Ther 253:981–986
Suh HH, Tseng LF, Li CH (1988) Beta-endorphin-(1-27) antagonizes beta-endorphin- but not morphine-, D-Pen2-D-Pen5-enkephalin- and U50, 488H-induced analgesia in mice. Neuropharmacology 27:957–963. https://doi.org/10.1016/0028-3908(88)90124-4
Suh HH, Fujimoto JM, Tseng LL-F (1989) Differential mechanisms mediating β-endorphin- and morphine-induced analgesia in mice. Eur J Pharmacol 168:61–70
Sullivan AF, McQuay HJ, Bailey D, Dickenson AH (1989) The spinal antinociceptive actions of morphine metabolites morphine-6-glucuronide and normorphine in the rat. Brain Res 482:219–224
Taha SA, Fields HL (2005) Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci 25:1193–1202. https://doi.org/10.1523/JNEUROSCI.3975-04.2005
Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL (2009) Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience 161:718–733. https://doi.org/10.1016/j.neuroscience.2009.03.057
Takagi H, Satoh M, Akaike A, Shibata T, Kuraishi Y (1977) The nucleus reticularis gigantocellularis of the medulla oblongata is a highly sensitive site in the production of morphine analgesia in the rat. Eur J Pharmacol 45:91–92. https://doi.org/10.1016/0014-2999(77)90064-4
Takagi H, Satoh M, Akaike A, Shibata T, Yajima H, Ogawa H (1978) Analgesic by enkephalins injected into the nucleus reticularis gigantocellularis of rat medulla oblongata. Eur J Pharmacol 49:113–116
Terenius L (1973) Stereospecific interaction between narcotic analgesics and a synaptic plasm a membrane fraction of rat cerebral cortex. Acta Pharmacol Toxicol 32:317–320. https://doi.org/10.1111/j.1600-0773.1973.tb01477.x
Terman GW, Liebeskind JC (1986) Relation of stress-induced analgesia to stimulation-produced analgesia. Ann N Y Acad Sci 467:300–308
Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC (1984) Intrinsic mechanisms of pain inhibition: activation by stress. Science 226:1270–1277. https://doi.org/10.1126/science.6505691
Terman GW, Penner ER, Liebeskind JC (1985) Stimulation-produced and stress-induced analgesia: cross-tolerance between opioid forms. Brain Res 360:374–378
Terman GW, Morgan MJ, Liebeskind JC (1986) Opioid and non-opioid stress analgesia from cold water swim: importance of stress severity. Brain Res 372:167–171
Terner J, Barrett A, Grossell E, Picker M (2002) Influence of gonadectomy on the antinociceptive effects of opioids in male and female rats. Psychopharmacology 163:183–193
Terner JM, Barrett AC, Cook CD, Picker MJ (2003a) Sex differences in (−)-pentazocine antinociception: comparison to morphine and spiradoline in four rat strains using a thermal nociceptive assay. Behav Pharmacol 14:77–85
Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ (2003b) Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain 106:381–391
Terskiy A, Wannemacher KM, Yadav PN, Tsai M, Tian B, Howells RD (2007) Search of the human proteome for endomorphin-1 and endomorphin-2 precursor proteins. Life Sci 81(23–24):1593–1601. https://doi.org/10.1016/j.lfs.2007.09.025
Thompson RC, Mansour A, Akil H, Watson SJ (1993) Cloning and pharmacological characterization of a rat μ opioid receptor. Neuron 11:903–913
Tian J-H, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, Han J-S (1997) Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol 120:676–680
Tobaldini G, Reis RA, Sardi NF, Lazzarim MK, Tomim DH, Lima MMS, Fischer L (2018) Dopaminergic mechanisms in periaqueductal gray-mediated antinociception. Behav Pharmacol 29:225–233. https://doi.org/10.1097/FBP.0000000000000346
Tseng LF, Collins KA (1991) Different mechanisms mediating tail-flick inhibition induced by beta-endorphin, DAMGO and morphine from ROb and GiA in anesthetized rats. J Pharmacol Exp Ther 257:530–538
Tseng LF, Fujimoto JM (1985) Differential actions of intrathecal naloxone on blocking the tail-flick inhibition induced by intraventricular beta-endorphin and morphine in rats. J Pharmacol Exp Ther 232:74–79
Tseng LL, Suh HH (1989) Intrathecal [Met5]enkephalin antibody blocks analgesia induced by intracerebroventricular beta-endorphin but not morphine in mice. Eur J Pharmacol 173:171–176. https://doi.org/10.1016/0014-2999(89)90515-3
Tseng LLF, Tang R (1989) Differential actions of the blockade of spinal opioid, adrenergic and serotonergic receptors on the tail-flick inhibition induced by morphine microinjected into dorsal raphe and central gray in rats. Neuroscience 33:93–100
Tseng LF, Tang R (1990) Different mechanisms mediate beta-endorphin- and morphine-induced inhibition of the tail-flick response in rats. J Pharmacol Exp Ther 252:546–551
Tseng LF, Tang RR (1992) Pentobarbital attenuates antinociception induced by i.c.v. morphine but not β-endorphin in the mouse. Eur J Pharmacol 214:175–180
Tseng LF, Wang Q (1992) Forebrain sites differentially sensitive to beta-endorphin and morphine for analgesia and release of Met-enkephalin in the pentobarbital-anesthesized rat. J Pharmacol Exp Ther 261:1028–1036
Tseng L-F, Higgins MJ, Hong J-S, Hudson PM, Fujimoto JM (1985) Release of immunoreactive met-enkephalin from the spinal cord by intraventricular β-endorphin but not morphine in anesthetized rats. Brain Res 343:60–69
Tseng LF, Narita M, Suganuma C, Mizoguchi H, Ohsawa M, Nagase H, Kampine JP (2000) Differential antinociceptive effects of endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther 292:576–583
Tsou K, Jang CS (1964) Studies on the site of analgesic action of morphine by intracerebral micro-injection. Sci Sin 13:1099–1109
Tung AS, Yaksh TL (1982) In vivo evidence for multiple opiate receptors mediating analgesia in the rat spinal cord. Brain Res 247:75–83
Uhl GR, Childers S, Pasternak G (1994) An opiate-receptor gene family reunion. Trends Neurosci 17:89–93
Urban MO, Smith DJ (1993) Role of neurotensin in the nucleus raphe magnus in opioid-induced antinociception from the periaqueductal gray. J Pharmacol Exp Ther 265:580–586
Van Bockstaele EJ, Pieribone VA, Aston-Jones G (1989) Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. J Comp Neurol 290:561–584
Van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT (1991) Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol 309:305–327. https://doi.org/10.1002/cne.903090303
van Praag H, Frenk H (1990) The role of glutamate in opiate descending inhibition of nociceptive spinal reflexes. Brain Res 524:101–105
Vaughan CW, Bagley EE, Drew GM, Schuller A, Pintar JE, Hack SP, Christie MJ (2003) Cellular actions of opioids on periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1. Br J Pharmacol 139:362–367. https://doi.org/10.1038/sj.bjp.0705261
Wamsley JK, Zarbin MA, Birdsall NJM, Kuhar MJ (1980) Muscarinic cholinergic receptors: autoradiographic localization of high and low affinity agonist binding sites. Brain Res 200:1–12
Wang JB, Imai Y, Eppler CM, Gregor P, Spivak CE, Uhl GR (1993) mu opiate receptor: cDNA cloning and expression. Proc Natl Acad Sci 90:10230–10234
Wang J-B, Johnson PS, Persico AM, Hawkins AL, Griffin CA, Uhl GR (1994a) Human μ opiate receptor: cDNA and genomic clones, pharmacologic characterization and chromosomal assignment. FEBS Lett 338:217–222
Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR (1994b) cDNA Cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348:75–79
Ward SJ, Takemori AE (1983) Relative involvement of receptor subtypes in opioid-induced inhibition of gastrointestinal transit in mice. J Pharmacol Exp Ther 224:359–363
Warlow SM, Naffziger EE, Berridge KC (2020) The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain. Nat Commun 11:2716. https://doi.org/10.1038/s41467-020-16407-1
Watkins L, Mayer D (1982) Organization of endogenous opiate and nonopiate pain control systems. Science 216:1185–1192
Watkins LR, Cobelli DA, Faris P, Aceto MD, Mayer DJ (1982a) Opiate vs non-opiate footshock-induced analgesia (FSIA): the body region shocked is a critical factor. Brain Res 242:299–308. https://doi.org/10.1016/0006-8993(82)90313-4
Watkins LR, Cobelli DA, Newsome HH, Mayer DJ (1982b) Footshock induced analgesia in dependent neither on pituitary nor sympathetic activation. Brain Res 245:81–96
Webster VA, Griffiths EC, Slater P (1983) Antinociceptive effects of thyrotrophin-releasing hormone and its analogues in the rat periaqueductal grey region. Neurosci Lett 42:67–70. https://doi.org/10.1016/0304-3940(83)90423-8
Wick MJ, Minnerath SR, Lin X, Elde R, Law P-Y, Loh HH (1994) Isolation of a novel cDNA encoding a putative membrane receptor with high homology to the cloned μ, δ, and κ opioid receptors. Mol Brain Res 27:37–44
Wiklund L, Behzadi G, Kalén P, Headley PM, Nicolopoulos LS, Parsons CG, West DC (1988) Autoradiographic and electrophysiological evidence for excitatory amino acid transmission in the periaqueductal gray projection to nucleus raphe magnus in the rat. Neurosci Lett 93:158–163
Winokur A, Utiger RD (1974) Thyrotropin-releasing hormone: regional distribution in rat brain. Science 185:265–267
Wise H (2012) The roles played by highly truncated splice variants of G protein-coupled receptors. J Mol Signal 7(1):13. https://doi.org/10.1186/1750-2187-7-13
Wolozin BL, Pasternak GW (1981) Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci 78:6181–6185
Wong C-L (1987a) The effect of gonadectomy on antinociception induced in mice by swim stress. Eur J Pharmacol 142:159–161
Wong CL (1987b) Sex difference in naloxone antagonism of swim stress-induced antinociception in mice. Methods Find Exp Clin Pharmacol 9:275–278
Wong C-L (1988) Effect of oestradiol replacement on swim-induced antinociception in ovariectomized mice. Clin Exp Pharmacol Physiol 15:799–802
Woolf NJ, Butcher LL (1989) Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull 23:519–540. https://doi.org/10.1016/0361-9230(89)90197-4
Xu J, Xu M, Hurd YL, Pasternak GW, Pan YX (2009) Isolation and characterization of new exon 11-associated N-terminal splice variants of the human mu opioid receptor gene. J Neurochem 108:962–972. https://doi.org/10.1111/j.1471-4159.2008.05833.x
Xu J, Xu M, Rossi GC, Pasternak GW, Pan YX (2011) Identification and characterization of seven new exon 11-associated splice variants of the rat μ opioid receptor gene, OPRM1. Mol Pain 7:9. https://doi.org/10.1186/1744-8069-7-9
Xu J et al (2013) Stabilization of the μ-opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J Biol Chem 288:21211–21227. https://doi.org/10.1074/jbc.M113.458687
Xu J, Lu Z, Xu M, Pan L, Deng Y, Xie X, Liu H, Ding S, Hurd YL, Pasternak GW, Klein RJ, Cartegni L, Zhou W, Pan YX (2014a) A heroin addiction severity-associated intronic single nucleotide polymorphism modulates alternative pre-mRNA splicing of the μ opioid receptor gene OPRM1 via hnRNPH interactions. J Neurosci 34(33):11048–11066
Xu J et al (2014b) Differential expressions of the alternatively spliced variant mRNAs of the micro opioid receptor gene, OPRM1, in brain regions of four inbred mouse strains. PLoS ONE 9:e111267. https://doi.org/10.1371/journal.pone.0111267
Xu J, Faskowitz AJ, Rossi GC, Xu M, Lu Z, Pan Y-X, Pasternak GW (2015) Stabilization of morphine tolerance with long-term dosing: association with selective upregulation of mu-opioid receptor splice variant mRNAs. Proc Natl Acad Sci 112:279–284
Xu J et al (2017) Alternatively spliced mu opioid receptor C termini impact the diverse actions of morphine. J Clin Investig 127:1561–1573
Yaksh TL (1979) Direct evidence that spinal serotonin and noradrenaline terminals mediate the spinal antinociceptive effects of morphine in the periaqueductal gray. Brain Res 160:180–185
Yaksh TL (1981) Spinal opiate analgesia: characteristics and principles of action. Pain 11:293–333
Yaksh TL (1984) Multiple opioid receptor systems in brain and spinal cord: Part 2. Eur J Anaesthesiol 1:201–243
Yaksh TL, Rudy TA (1978) Narcotic analgesics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain 4:299–359. https://doi.org/10.1016/0304-3959(77)90145-2
Yaksh TL, Yeung JC, Rudy TA (1976) Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114:83–103
Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI (1993) Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci 90:6736–6740
Yeomans DC, Proudfit HK (1992) Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience 49:681–691. https://doi.org/10.1016/0306-4522(92)90236-u
Yeomans DC, Clark FM, Paice JA, Proudfit HK (1992) Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain 48:449–461. https://doi.org/10.1016/0304-3959(92)90098-v
Yeung JC, Rudy TA (1980a) Sites of antinociceptive action of systemically injected morphine: involvement of supraspinal loci as revealed by intracerebroventricular injection of naloxone. J Pharmacol Exp Ther 215:626–632
Yeung JC, Rudy TA (1980b) Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J Pharmacol Exp Ther 215:633–642
Yoburn BC, Truesdell LS, Kest B, Inturrisi CE, Bodnar RJ (1987) Chronic opioid antagonist treatment facilitates nonopioid, stress-induced analgesia. Pharmacol Biochem Behav 27:525–527
Zadina JE, Hackler L, Ge L-J, Kastin AJ (1997) A potent and selective endogenous agonist for the µ-opiate receptor. Nature 386:499–502
Zastawny RL, George SR, Nguyen T, Cheng R, Tsatsos J, Briones-Urbina R, O’Dowd BF (1994) Cloning, characterization, and distribution of a mu-opioid receptor in rat brain. J Neurochem 62:2099–2105. https://doi.org/10.1046/j.1471-4159.1994.62062099.x
Zhang AZ, Pasternak GW (1980) mu- and delta-opiate receptors: correlation with high and low affinity opiate binding sites. Eur J Pharmacol 67:323–324. https://doi.org/10.1016/0014-2999(80)90518-x
Zhang A-Z, Pasternak GW (1981a) Ontogeny of opioid pharmacology and receptors: high and low affinity site differences. Eur J Pharmacol 73:29–40
Zhang AZ, Pasternak GW (1981b) Opiates and enkephalins: a common binding site mediates their analgesic actions in rats. Life Sci 29:843–851. https://doi.org/10.1016/0024-3205(81)90041-2
Zhang RX, Zhang M, Li A, Pan L, Berman BM, Ren K, Lao L (2013) DAMGO in the central amygdala alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia. Neuroscience 252:359–366. https://doi.org/10.1016/j.neuroscience.2013.08.030
Zhu J, Chen C, Xue J-C, Kunapuli S, DeRiel JK, Liu-Chen L-Y (1995) Cloning of a human κ opioid receptor from the brain. Life Sci 56:PL201–PL207
Zorrilla EP, Koob GF (2013) Amygdalostriatal projections in the neurocircuitry for motivation: a neuroanatomical thread through the career of Ann Kelley. Neurosci Biobehav Rev 37:1932–1945. https://doi.org/10.1016/j.neubiorev.2012.11.019
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The authors of the submitted manuscript have contributed equally to the research and writing of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors of the submitted manuscript have no conflicts of interest to declare.
Consent for publication
Both authors consent to the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rossi, G.C., Bodnar, R.J. Interactive Mechanisms of Supraspinal Sites of Opioid Analgesic Action: A Festschrift to Dr. Gavril W. Pasternak. Cell Mol Neurobiol 41, 863–897 (2021). https://doi.org/10.1007/s10571-020-00961-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00961-9