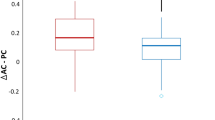
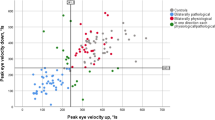
Abstract
Objective
To investigate oculomotor impairment in subtypes of progressive supranuclear palsy (PSP) and its associations with clinical features and regional brain volumes in PSP.
Methods
We compared the video-oculography (VOG) findings of 123 PSP patients, consisting of 66 PSP-Richardson syndrome (PSP-RS), 28 PSP-parkinsonism (PSP-P), and 29 PSP-progressive gait freezing (PSP-PGF), along with 80 Parkinson’s disease (PD) patients. We also investigated the associations of the VOG results with clinical features (disease duration, PSP rating scales [PSPRS] scores for dysphagia and postural stability) in the subtypes of PSP patients and with regional volumes in the brainstem, including the midbrain, pons, medulla, and the superior cerebellar peduncle (SCP), among the patients who had MRI images at the time of VOG (30 PSP).
Results
All of the three subtypes of PSP patients showed slower vertical saccades and smooth pursuit than that of the PD patients (adjusted p < 0.05). Among the PSP subtypes, saccadic peak velocity, saccadic accuracy, and pursuit gain were significantly decreased in patients with the PSP-RS compared to those with the PSP-PGF (adjusted p < 0.05). In multiple linear regression model, vertical saccadic velocity, latency, accuracy, and pursuit gain were associated with the PSPRS score for dysphagia (adjusted p < 0.05), and a decrease in vertical saccadic speed and accuracy was associated with SCP atrophy (corrected p < 0.05).
Conclusions
This study demonstrated the severity of oculomotor dysfunction in differentiating the subtypes of PSP and its significant relationships with the dysphagia symptom and SCP volume in PSP.


Similar content being viewed by others
References
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord Off J Move Disord Soc 32(6):853–864. https://doi.org/10.1002/mds.26987
Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW (2003) Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 60(11):1766–1769. https://doi.org/10.1212/01.wnl.0000068011.21396.f4
Paviour DC, Price SL, Stevens JM, Lees AJ, Fox NC (2005) Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 64(4):675–679. https://doi.org/10.1212/01.Wnl.0000151854.85743.C7
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51(6):745–752. https://doi.org/10.1136/jnnp.51.6.745
Kim JS, Oh SY, Lee SH, Kang JH, Kim DU, Jeong SH, Choi KD, Moon IS, Kim BK, Oh HJ, Kim HJ (2012) Randomized clinical trial for apogeotropic horizontal canal benign paroxysmal positional vertigo. Neurology 78(3):159–166. https://doi.org/10.1212/WNL.0b013e31823fcd26
Park HK, Kim JS, Strupp M, Zee DS (2013) Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol 260(6):1576–1582. https://doi.org/10.1007/s00415-013-6837-y
Chen AL, Riley DE, King SA, Joshi AC, Serra A, Liao K, Cohen ML, Otero-Millan J, Martinez-Conde S, Strupp M, Leigh RJ (2010) The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front Neurol 1:147. https://doi.org/10.3389/fneur.2010.00147
Vintonyak O, Gorges M, Müller HP, Pinkhardt EH, Ludolph AC, Huppertz HJ, Kassubek J (2017) Patterns of eye movement impairment correlate with regional brain atrophy in neurodegenerative Parkinsonism. Neuro-degenerative diseases 17(4–5):117–126. https://doi.org/10.1159/000454880
Pinkhardt EH, Jurgens R, Becker W, Valdarno F, Ludolph AC, Kassubek J (2008) Differential diagnostic value of eye movement recording in PSP-parkinsonism, Richardson’s syndrome, and idiopathic Parkinson’s disease. J Neurol 255(12):1916–1925. https://doi.org/10.1007/s00415-009-0027-y
Leigh RJ, Zee DS (2015) The Neurology of Eye Movements, 5th edn. Oxford University Press, New York
Golbe LI, Ohman-Strickland PA (2007) A clinical rating scale for progressive supranuclear palsy. Brain J Neurol 130(Pt 6):1552–1565. https://doi.org/10.1093/brain/awm032
Bocchetta M, Iglesias JE, Chelban V, Jabbari E, Lamb R, Russell LL, Greaves CV, Neason M, Cash DM, Thomas DL, Warren JD, Woodside J, Houlden H, Morris HR, Rohrer JD (2019) Automated brainstem segmentation detects differential involvement in atypical Parkinsonian syndromes. J Move Disord. https://doi.org/10.14802/jmd.19030
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8(3):270–279. https://doi.org/10.1016/s1474-4422(09)70042-0
Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T (2007) Pathological tau burden and distribution distinguishes progressive supranuclear Palsy-Parkinsonism from Richardson’s syndrome. Brain J Neurol 130(Pt 6):1566–1576. https://doi.org/10.1093/brain/awm104
Büttner-Ennever JA, Büttner U (1978) A cell group associated with vertical eye movements in the rostral mesencephalic reticular formation of the monkey. Brain Res 151(1):31–47. https://doi.org/10.1016/0006-8993(78)90948-4
Horn AK, Buttner-Ennever JA (1998) Premotor neurons for vertical eye movements in the rostral mesencephalon of monkey and human: histologic identification by parvalbumin immunostaining. J Comp Neurol 392(4):413–427
Horn AK, Helmchen C, Wahle P (2003) GABAergic neurons in the rostral mesencephalon of the macaque monkey that control vertical eye movements. Ann NY Acad Sci 1004:19–28. https://doi.org/10.1196/annals.1303.003
Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S (2003) Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain J Neurol 126(Pt 6):1460–1473. https://doi.org/10.1093/brain/awg148
Kheradmand A, Zee DS (2011) Cerebellum and ocular motor control. Front Neurol 2:53. https://doi.org/10.3389/fneur.2011.00053
Watanabe M, Munoz DP (2011) Probing basal ganglia functions by saccade eye movements. Eur J Neurosci 33(11):2070–2090. https://doi.org/10.1111/j.1460-9568.2011.07691.x
Anderson TJ, MacAskill MR (2013) Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol 9(2):74–85. https://doi.org/10.1038/nrneurol.2012.273
Warnecke T, Oelenberg S, Teismann I, Hamacher C, Lohmann H, Ringelstein EB, Dziewas R (2010) Endoscopic characteristics and levodopa responsiveness of swallowing function in progressive supranuclear palsy. Move Disord Off J Move Disord Soc 25(9):1239–1245. https://doi.org/10.1002/mds.23060
Clark HM, Stierwalt JAG, Tosakulwong N, Botha H, Ali F, Whitwell JL, Josephs KA (2019) Dysphagia in Progressive Supranuclear Palsy. Dysphagia. https://doi.org/10.1007/s00455-019-10073-2
Esmonde T, Giles E, Gibson M, Hodges JR (1996) Neuropsychological performance, disease severity, and depression in progressive supranuclear palsy. J Neurol 243(9):638–643. https://doi.org/10.1007/bf00878659
Whitwell JL, Schwarz CG, Reid RI, Kantarci K, Jack CR Jr, Josephs KA (2014) Diffusion tensor imaging comparison of progressive supranuclear palsy and corticobasal syndromes. Parkinsonism Relat Disord 20(5):493–498. https://doi.org/10.1016/j.parkreldis.2014.01.023
Agosta F, Galantucci S, Svetel M, Lukic MJ, Copetti M, Davidovic K, Tomic A, Spinelli EG, Kostic VS, Filippi M (2014) Clinical, cognitive, and behavioural correlates of white matter damage in progressive supranuclear palsy. J Neurol 261(5):913–924. https://doi.org/10.1007/s00415-014-7301-3
Caso F, Agosta F, Volonte MA, Ferraro PM, Tiraboschi P, Copetti M, Valsasina P, Falautano M, Comi G, Falini A, Filippi M (2016) Cognitive impairment in progressive supranuclear Palsy-Richardson’s syndrome is related to white matter damage. Parkinsonism Relat Disord 31:65–71. https://doi.org/10.1016/j.parkreldis.2016.07.007
Whitwell JL, Avula R, Master A, Vemuri P, Senjem ML, Jones DT, Jack CR Jr, Josephs KA (2011) Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat Disord 17(8):599–605. https://doi.org/10.1016/j.parkreldis.2011.05.013
Zhang Y, Walter R, Ng P, Luong PN, Dutt S, Heuer H, Rojas-Rodriguez JC, Tsai R, Litvan I, Dickerson BC, Tartaglia MC, Rabinovici G, Miller BL, Rosen HJ, Schuff N, Boxer AL (2016) Progression of microstructural degeneration in progressive supranuclear palsy and corticobasal syndrome: a longitudinal diffusion tensor imaging study. PLoS ONE 11(6):e0157218. https://doi.org/10.1371/journal.pone.0157218
Agosta F, Caso F, Jecmenica-Lukic M, Petrovic IN, Valsasina P, Meani A, Copetti M, Kostic VS, Filippi M (2018) Tracking brain damage in progressive supranuclear palsy: a longitudinal MRI study. J Neurol Neurosurg Psychiatry 89(7):696–701. https://doi.org/10.1136/jnnp-2017-317443
Hoglinger GU, Schope J, Stamelou M, Kassubek J, Del Ser T, Boxer AL, Wagenpfeil S, Huppertz HJ (2017) Longitudinal magnetic resonance imaging in progressive supranuclear palsy: a new combined score for clinical trials. Move Disord Off J Move Disord Soc 32(6):842–852. https://doi.org/10.1002/mds.26973
Longoni G, Agosta F, Kostic VS, Stojkovic T, Pagani E, Stosic-Opincal T, Filippi M (2011) MRI measurements of brainstem structures in patients with Richardson’s syndrome, progressive supranuclear palsy-parkinsonism, and Parkinson’s disease. Move Disord Off J Move Disord Soc 26(2):247–255. https://doi.org/10.1002/mds.23293
Funding
This work was supported by Grant no. 0420193140 from the Seoul National University Hospital Research Fund.
Author information
Authors and Affiliations
Contributions
J-HC, MD: design of the study, data analysis, interpretation of data, drafted and revised the manuscript. HJK, PhD: data analysis, interpretation of data, and revision of manuscript. JH Shin, MD, PhD: interpretation of data and revision of manuscript. J-YL MD, PhD: design of the study, interpretation of data, and revision of manuscript. H-JK, MD, PhD: interpretation of data and revision of manuscript. J-MK, MD, PhD: interpretation of data and revision of manuscript. BJ, MD, PhD: conceptualization and design of study, data analysis, interpretation of data, and revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
HJK has received travel grant from International Parkinson and Movement Disorder Society and Korean Movement Disorder Society; research grant from the Seoul National University Hospital, New York University, and C-TRI. BJ has received grants from the International Parkinson and Movement Disorder Society, Peptron, and Abbvie Korea.
Ethical approval
This study was approved by the Ethical Committee of the Seoul National University, Seoul, Korea (No. 1904-062-1026). In addition, this study was conducted in accordance with the Helsinki Declaration.
Data access and responsibility statement
The corresponding author is able to provide the anonymized data of this study upon reasonable request from qualified investigators.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2020_10230_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1. Eye movements by disease duration in the patients with PSP. (A) Saccadic peak velocity, (B) Saccadic latency, (C) Saccadic accuracy, (D) Smooth pursuit gain. * p, Significant difference between the PSP-RS subtype and the PSP-P and PSP-PGF subtypes at 1, 2, and 3 years of the disease duration by Mann–Whitney test. * p-value < 0.05, ** < 0.01, and *** < 0.001. Abbreviations: PSP = Progressive supranuclear palsy; RS = Richardson’s syndrome; P = parkinsonism; PGF = progressive gait freezing. (TIF 55640 kb)
415_2020_10230_MOESM2_ESM.tif
Supplementary file2 Supplementary Fig. 2. Correlational analysis between the dysphagia PSP rating scale and eye movements in the patients with PSP. Pearson’s correlation test between the dysphagia PSP rating scale and eye movements in patients with PSP (n = 123). Abbreviations: PSP = Progressive supranuclear palsy. (TIF 1669 kb)
415_2020_10230_MOESM4_ESM.docx
Supplementary file4 Supplementary Table 2. Eye movements from 1 to 3 years of the disease duration in the patients with PSP (DOCX 34 kb)
415_2020_10230_MOESM5_ESM.docx
Supplementary file5 Supplementary Table 3. Correlational analysis between clinical variables and eye movements in the patients with PSP (DOCX 40 kb)
415_2020_10230_MOESM6_ESM.docx
Supplementary file6 Supplementary Table 4. Regional volumes in the brainstem and the superior cerebellar peduncle in the patients with PSP (DOCX 29 kb)
415_2020_10230_MOESM7_ESM.docx
Supplementary file7 Supplementary Table 5. Correlational analysis of the regional volumes in the brainstem and the superior cerebellar peduncle with eye movements in the patients with PSP (DOCX 31 kb)
415_2020_10230_MOESM8_ESM.docx
Supplementary file8 Supplementary Table 6. Multivariate analysis of the regional brain volumes with eye movements in the patients with PSP (DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Choi, JH., Kim, H., Shin, J.H. et al. Eye movements and association with regional brain atrophy in clinical subtypes of progressive supranuclear palsy. J Neurol 268, 967–977 (2021). https://doi.org/10.1007/s00415-020-10230-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10230-w