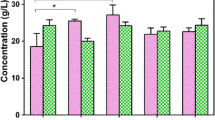
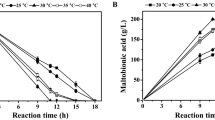
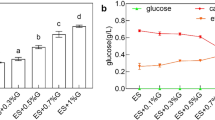
Abstract
β-poly(L-malic acid) (PMLA) has attracted industrial interest for its potential applications in medicine and other industries. For a sustainable PMLA production, it requires replacing/reducing the CaCO3 usage, since the residual CaCO3 impeded the cells’ utilization, and a large amount of commercially useless gypsum was accumulated. In this study, it was found that more glucose was converted into CO2 using soluble alkalis compared with CaCO3 usage. Moreover, since the high ion strength and respiration effect of soluble alkalis also inhibited PMLA production, they could not effectively replace CaCO3. Furthermore, comparing the fermentations with different neutralizers (soluble alkali vs. CaCO3), it was found that the differential genes are mainly involved in the pathway of starch and sucrose metabolism, pentose and glucuronate interconversions, histidine metabolism, ascorbate and aldarate metabolism, and phagosome. In detail, in the case with CaCO3, 562 genes were downregulated and 262 genes were upregulated, and especially, those genes involved in energy production and conversion were downregulated by 26.7%. Therefore, the irreplaceability of CaCO3 was caused by its effect on the PMLA metabolic pathway rather than its usage as neutralizer. Finally, a combined pH shift control strategy with CaCO3 addition was developed. After the fermentation, 64.8 g/L PMLA and 38.9 g/L biomass were obtained with undetectable CaCO3 and less CO2 emission.
Key points
• The effect of CaCO3 on PMLA metabolic pathway resulted in its irreplaceability.
• A pH shift control strategy with CaCO 3 addition was developed.
• Undetectable CaCO 3 and less CO 2 emission were detected with the new strategy.

Graphical abstract








Similar content being viewed by others
References
Arif M, Dong QJ, Raja MA, Zeenat S, Chi Z, Liu CG (2018) Development of novel pH-sensitive thiolated chitosan/PMLA nanoparticles for amoxicillin delivery to treat Helicobacter pylori. Mater Sci Eng C 83:17–24
Ataei SA, Vasheghani-Farahani E (2008) In situ separation of lactic acid from fermentation broth using ion exchange resins. J Ind Microbiol Biotechnol 35:1229–1233
Cao WF, Luo JQ, Zhao J, Qiao CS, Ding LH, Qi BK, Su Y, Wan YH (2012) Intensification of β-poly(L-malic acid) production by Aureobasidium pullulans ipe-1 in the late exponential growth phase. J Ind Microbiol Biotechnol 39:1073–1080
Cao W, Qi B, Zhao J, Qiao C, Su Y, Wan Y (2013) Control strategy of pH, dissolved oxygen concentration and stirring speed for enhancing β-poly(malic acid) production by Aureobasidium pullulans ipe-1. J Chem Technol Biotechnol 88:808–817
Cao W, Chen X, Luo J, Yin J, Qiao C, Wan Y (2016) High molecular weight β-poly (L-malic acid) produced by A. pullulans with Ca2+ added repeated batch culture. Int J Biol Macromol 85:192–199
Cao W, Wang Y, Luo J, Yin J, Xing J, Wan Y (2018) Effectively converting carbon dioxide into succinic acid under mild pressure with Actinobacillus succinogenes by an integrated fermentation and membrane separation process. Bioresour Technol 266:26–33
Cao W, Wang Y, Shen F, Luo J, Yin J, Qiao C, Wan Y (2019) Efficient β-poly(L-malic acid) production from Jerusalem artichoke by Aureobasidium pullulans ipe-1 immobilized in luffa sponge matrices. Bioresour Technol 288:121497
Cao W, Cao W, Shen F, Luo J, Yin J, Qiao C, Wan Y (2020) Membrane-assisted β-poly(L-malic acid) production from bagasse hydrolysates by Aureobasidium pullulans ipe-1. Bioresour Technol 295:122260
Feng J, Yang J, Yang W, Chen J, Jiang M, Zou X (2018) Metabolome- and genome-scale model analyses for engineering of Aureobasidium pullulans to enhance polymalic acid and malic acid production from sugarcane molasses. Biotechnol Biofuels 11:94
Frej KAK, Hjorth RA (2018) Expanded bed adsorption. Biopharmaceutical Processing, (Eds.), Elsevier, 269-277
Gibbs P, Seviour R (1996) Does the agitation rate and/or oxygen saturation influence exopolysaccharide production by Aureobasidium pullulans in batch culture? Appl Microbiol Biotechnol 46:503–510
Gupta P, Paul S (2014) Solid acids: green alternatives for acid catalysis. Catal Today 236:153–170
Israel LL, Braubach O, Galstyan A, Chiechi A, Shatalova ES, Grodzinski Z, Ding H, Black KL, Ljubimova JY, Holler E (2019) A combination of tri-leucine and angiopep-2 drives a polyanionic polymalic acid nanodrug platform across the blood–brain barrier. ACS Nano 13:1253–1271
Lee B, Maurer T, Kalbitzer H, Holler E (1999) β-Poly (L-malate) production by Physarum polycephalum. Appl Microbiol Biotechnol 52:415–420
Li J, Chase HA (2009) Use of expanded bed adsorption to purify flavonoids from Ginkgo biloba L. J Chromatog A 1216:8759–8770
Li Q, Wang D, Hu G, Xing J, Su Z (2011) Integrated bioprocess for high-efficiency production of succinic acid in an expanded-bed adsorption system. Biochem Eng J 56:150–157
Li YC, Xie CY, Yang BX, Tang YQ, Wu B, Sun ZY, Gou M, Xia ZY (2019) Comparative transcriptome analysis of recombinant industrial Saccharomyces cerevisiae strains with different xylose utilization pathways. Appl Biochem Biotechnol 189:1007–1019
Liu S, Steinbüchel A (1996) Investigation of poly (β-L-malic acid) production by strains of Aureobasidium pullulans. Appl Microbiol Biotechnol 46:273–278
Liu S, Steinbüchel A (1997) Production of polymalic acid by Aureobasidium pullulans CBS591175 and DSM2404 in 2L and 20L fermenters. Chin J Biotechnol 13:279–283
Ma Y, Wang GY, Liu GL, Wang ZP, Chi ZM (2013) Overproduction of poly(β-malic acid) (PMA) from glucose by a novel Aureobasidium sp. P6 strain isolated from mangrove system. Appl Microbiol Biotechnol 97:8931–8939
Manitchotpisit P, Skory CD, Peterson SW, Price NPJ, Vermillion KE, Leathers TD (2011) Poly (β-L-malic acid) production by diverse phylogenetic clades of Aureobasidium pullulans. J Ind Microbiol Biotechnol 39:125–132
Pathapati T, Rütze DN, den Boer P, de Wit P, Zaalberg M (2018) Expanded bed adsorption of γ-aminobutyric acid from E. coli broth by CS16GC and IRC747 resins. Chem Eng Technol 41:2427–2434
Sheng L, Tong Q, Ma M (2016) Why sucrose is the most suitable substrate for pullulan fermentation by Aureobasidium pullulans CGMCC1234? Enzym Microb Technol 92:49–55
Sun D, Li H, Song D, Zhang L, Zhao X, Xu X (2019a) Genome, transcriptome and fermentation analyses of Lactobacillus plantarum LY-78 provide new insights into the mechanism of phenyllactate biosynthesis in lactic acid bacteria. Biochem Bioph Res Co 519:351–357
Sun ZG, Wang MQ, Wang YP, Xing S, Hong KQ, Chen YF, Guo XW, Xiao DG (2019b) Identification by comparative transcriptomics of core regulatory genes for higher alcohol production in a top-fermenting yeast at different temperatures in beer fermentation. Appl Microbiol Biotechnol 103:4917–4929
Wang YK, Chi Z, Zhou HX, Liu GL, Chi ZM (2015) Enhanced production of Ca2+-polymalate (PMA) with high molecular mass by Aureobasidium pullulans var. pullulans MCW. Microb Cell Factories 14:115
Wei P, Cheng C, Lin M, Zhou Y, Yang ST (2017) Production of poly(malic acid) from sugarcane juice in fermentation by Aureobasidium pullulans: kinetics and process economics. Bioresour Technol 224:581–589
West TP (2017) Microbial production of malic acid from biofuel-related coproducts and biomass. Fermentation 3:14
Xia J, Liu X, Xu J, Xu J, Wang X, Li X (2017a) Simultaneously enhanced production of poly(β-malic acid) and pullulan using a dissolved oxygen shift (DO-shift) control strategy. J Chem Technol Biotechnol 92:1464–1471
Xia J, Xu J, Liu X, Xu J, Wang X, Li X (2017b) Economic co-production of poly(malic acid) and pullulan from Jerusalem artichoke tuber by Aureobasidium pullulans HA-4D. BMC Biotechnol 17:20
Xue B, Tong X, Sun Y (2003) Polydisperse model for the hydrodynamics of expanded-bed adsorption systems. AICHE J 49:2510–2518
Yu H, Liu B, Luo J, Cao W, Qiao C, Wan Y (2018) Toward understanding the key enzymes involved in β-poly (L-malic acid) biosynthesis by Aureobasidium pullulans ipe-1. Eng Life Sci 18:379–386
Zeng W, Zhang B, Li M, Ding S, Chen G, Liang Z (2019) Development and benefit evaluation of fermentation strategies for poly(malic acid) production from malt syrup by Aureobasidium melanogenum GXZ-6. Bioresour Technol 274:479–487
Zhang K, Yang ST (2015) In situ recovery of fumaric acid by intermittent adsorption with IRA-900 ion exchange resin for enhanced fumaric acid production by Rhizopus oryzae. Biochem Eng J 96:38–45
Zhang H, Cai J, Dong J, Zhang D, Huang L, Xu Z, Cen P (2011) High-level production of poly (β-L-malic acid) with a new isolated Aureobasidium pullulans strain. Appl Microbiol Biotechnol 92:295–303
Zhu H, Sadiq FA, Li Y, Yang S, Zhou F (2018) Application of ion-exchange resin as solid acid for buffer-free production of γ-aminobutyric acid using Enterococcus faecium cells. LWT-Food Sci Technol 98:341–348
Zou X, Tu G, Zan Z (2014) Cofactor and CO2 donor regulation involved in reductive routes for polymalic acid production by Aureobasidium pullulans CCTCC M2012223. Bioprocess Biosyst Eng 37:2131–2136
Zou X, Yang J, Tian X, Guo M, Li Z, Li Y (2016) Production of polymalic acid and malic acid from xylose and corncob hydrolysate by a novel Aureobasidium pullulans YJ 6–11 strain. Process Biochem 51:16–23
Zou X, Cheng C, Feng J, Song X, Lin M, Yang ST (2019) Biosynthesis of polymalic acid in fermentation: advances and prospects for industrial application. Crit Rev Biotechnol 39:408–421
Contributions
WC (Weifeng Cao) conceived and designed research. CQ and YW conducted experiments. FS contributed new reagents or analytical tools. WC analyzed data. WC (Weifeng Cao) and JL wrote the manuscript. All authors read and approved the manuscript.
Funding
The authors thank the Beijing Natural Science Foundation, China (No. 5182025); the National Natural Science Foundation of China, China (No. 21406240); and the National High Technology Research and Development Program of China (Nos. 2015AA021002 and 2014AA021005) for the financial supports.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 126 kb)
Rights and permissions
About this article
Cite this article
Cao, W., Cao, W., Shen, F. et al. A sustainable pH shift control strategy for efficient production of β-poly(L-malic acid) with CaCO3 addition by Aureobasidium pullulans ipe-1. Appl Microbiol Biotechnol 104, 8691–8703 (2020). https://doi.org/10.1007/s00253-020-10815-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10815-5