Abstract
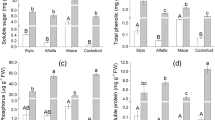
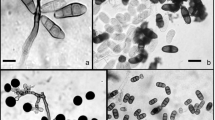
To address correlations between population sizes of microbes on the leaf surfaces and leaf morphological and physicochemical properties, various leaf morphological and physicochemical features as possible predictors of microbial population sizes on the leaf surfaces of four Napier grass cultivars were assessed. Results indicated microbes except for lactic acid bacteria (LAB) preferred to colonize the leaf surfaces bearing trichomes, and their population sizes were significantly correlated with trichomes, especially for yeasts. The population sizes of microbes were positively correlated with soluble sugar content (p < 0.05). Furthermore, no significant correlation was found between population sizes of microbes and wax content, except for yeasts. The multivariate regression trees (MRT) analysis showed different genotypes of leaf–microbe system could be characterized by four-leaf attributes with soluble sugar of leaf tissues being the primary explanatory attribute. Leaves with soluble sugar content below 9.72 mg g−1 fresh weight (FW) were rarely colonized. For leaves with soluble sugar content above 9.72 mg g−1 FW, water content was the next explanatory leaf attribute, followed by wax content on the leaf surfaces. Leaves with higher water content (> 73%) were more colonized, and small microbial population was associated with higher wax content (> 10.66 mg g−1 dry matter). In conclusion, leaf chemical attributes have a higher contribution than morphological structure properties in determining population sizes of microbes on the leaf surfaces. The exuded soluble sugar and protein promote the development of microbial populations. For different genotypes of leaf–microbe system, the relationship between microbial abundance on their leaf surfaces and leaf morphological structure or physicochemical properties may be predicted by the MRT. Population sizes of microbes are primarily influenced by soluble sugar content under the water-rich conditions.







Similar content being viewed by others
References
Beattie GA (2002) Leaf surface wax and the process of leaf colonization by microorganisms. In: Lindow SE, Hecht-Poinar EI, Elliott V (eds) Phyllophere microbiology. APS Press, St. Paul, Minn, pp 3–26
Bewick TA, Shilling DG, Querns R (1993) Evaluation of epicuticular wax removal from whole leaves with chloroform. Weed Technol 7(3):706–716
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, Berlin, pp 99–109
Chen XZ, Zhuang YF, Dong ZX, Zhang JG (2017) Factors influencing the distribution of lactic acid bacteria on Pennisetum grasses. Grassl Sci 63(3):150–158
Correa OS, Romero AM, Montecchia MS, Soria MA (2007) Tomato genotype and Azospirillum inoculation modulate the changes in bacterial communities associated with roots and leaves. J Appl Microbiol 102:781–786
De’ath G (2002) Multivariate regression trees: a technique for modeling species-environment relations. Ecology 83(4):1105–1117
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28:350–356
Ehleringer JR, Mooney HA (1978) Leaf hairs: effects on physiological activity and adaptive value to a desert shrub. Oecologia 37:183–200
Fiala V, Glad C, Martin M, Jolivet E, Derridj S (1990) Occurrence of soluble carbohydrates on the phylloplane of maize (Zea mays L.): variations in relation to leaf heterogeneity and position on the plant. New Phytol 115:609–615
Hartmann A, Schikora A (2012) Quorum sensing of bacteria and trans-kingdom interactions of N-Acyl homoserine lactones with eukaryotes. J Chem Ecol 38:704–713
Hunter PJ, Hand P, Pink D, Whipps JM, Bending GD (2010) Both leaf properties and microbe-microbe interactions influence within-cultivars variation in bacterial population diversity and structure in the lettuce (Lactuca cultivars) phyllosphere. Appl Environ Microb 76:8117–8125
Jacques M (1996) The effect of leaf age and position on the dynamics of microbial populations on aerial plant surfaces. In: Morris CE, Nicot PC, Nguyen CN (eds) Aerial plant surface microbiology. Plenum Press, New York, pp 233–248
Jeffree C (2006) The fine structure of the plant cuticle. In: Riederer M, Müller C (eds) Biology of plant cuticle. Blackwell, Oxford, pp 11–125
Karabourniotis G, Kyparissis A, Manetas Y (1993) Leaf hairs of Olea europaea L. protect underlying tissue against ultraviolet-B radiation damage. Environ Exp Bot 33:341–345
Klerks MM, Franz E, van Gent-Pelzer M, Zijlstra C, van Bruggen AH (2007) Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. ISME J 1:620–631
Knoll D, Schreiber L (2000) Plant-microbe interactions: wettability of ivy (Hedera helix L.) leaf surfaces in relation to colonization by epiphytic microorganisms. Microb Ecol 40:33–42
Knowles V, Plaxton W (2013) Quantification of total and soluble inorganic phosphate. Bioprotocol 3:e890
Leroy C, Jauneau A, Quilichini A, Dejean A, Orivel J (2008) Comparison between the anatomical and morphological structure of leaf blades and foliar domatia in the Ant-plant Hirtella physophora (Chrysobalanaceae). Ann Bot Lond 101:501–507
Marcell LM, Beattie GA (2002) Effect of leaf surface wax on leaf colonization by Pantoea agglomerans and Clavibacter michiganensis. Mol Plant Microbe 15:1236–1244
Ni Y, Sun ZY, Huang XZ, Huang CS, Guo YJ (2015) Variations of cuticular wax in mulberry trees and their effects on gas exchange and post-harvest water loss. Acta Physiol Plant 37:112
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Reisberg EE, Hildebrandt U, Riederer M, Hentschel U (2013) Distinct phyllosphere bacterial communities on Arabidopsis wax mutant leaves. PLoS ONE 8:e78613
Remus-Emsermann MN, Lucker S, Muller DB, Potthoff E, Daims H, Vorholt JA (2014) Spatial distribution analyses of natural phyllosphere-colonizing bacteria on Arabidopsis thaliana revealed by fluorescence in situ hybridization. Environ Microbiol 16:2329–2340
Schönherr J, Baur P (1996) Cuticle permeability studies: a model for estimating leaching of plant metabolites to leaf surfaces. In: Morris CE, Nicot PC, Nguyen C (eds) Aerial plant surface microbiology. Plenum Press, New York, pp 1–23
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbial 10(12):828–840
Waterman P, Mole S (1994) Analysis of phenolic plant metabolites. In: Lawton JH, Likens GE (eds) Methods in ecology. Blackwell Scientific Publication, Oxford, pp 66–103
Whipps JM, Hand P, Pink D, Bending GD (2008) Phyllosphere microbiology with special reference to diversity and plant genotype. J Appl Microbiol 105:1744–1755
Yadav RKP, Karamanoli K, Vokou D (2005) Bacterial colonization of the phyllosphere of mediterranean perennial cultivars as influenced by leaf structural and chemical features. Microb Ecol 50:185–196
Yu AO, Leveau JHJ, Marco ML (2020) Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Env Microbiol Rep 12(1):16–29
Acknowledgements
This work was financed by the National Natural Science Foundation of China (Nos. 31672486 and 31971764). We are grateful to Weiwei Zhang and Pengyao Lu for their assistance in measuring contact angle.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Communicated by Erko stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, G., Xu, L., Yin, X. et al. Microbial colonization on the leaf surfaces of different genotypes of Napier grass. Arch Microbiol 203, 335–346 (2021). https://doi.org/10.1007/s00203-020-02025-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02025-4