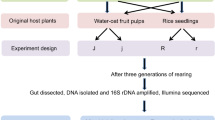
Abstract
Gut microbiota plays vital roles in the development, evolution and environmental adaptation of the host insects. The brown planthopper (BPH) is one of the most destructive pests of rice, but little is known about its gut microbiota. In this study, we investigated the gut bacterial communities in two BPH populations feeding on susceptible and resistant rice varieties by high-throughput amplicon sequencing. Our results revealed that the gut bacterial communities in BPH were species diverse. A total of 29 phyla and 367 genera were captured, with Proteobacteria and Acinetobacter being the most prominent phylum and genus, respectively. Comparative analysis showed that significant differences in the profile of gut bacterial communities existed between the two BPH populations. The species richness detected in the population feeding on the resistant rice variety was significantly higher than that in the population rearing on the susceptible rice variety. Although the most dominant gut bacteria at all taxonomic levels showed no significant differences between the two BPH populations, the relative abundances of two subdominant phyla (Firmicutes and Bacteroidetes) and two subdominant classes (Bacteroidia and Clostridia) were significantly different. FAPROTAX analysis further indicated that host rice varieties might induce changes of the gut bacterial flora in BPH, as significant differences in five metabolism-related functional categories (fermentation, methylotrophy, xylanolysis, nitrate reduction and ureolysis) were detected between the two BPH populations. Our results are informative for studies which focused on the interactions between BPH and its symbiotic microbes and could also provide the basis of future BPH biological management.





Similar content being viewed by others
References
Alagar M, Suresh S, Samiyappan R, Saravanakumar D (2007) Reaction of resistant and susceptible rice genotypes against brown planthopper (Nilaparvata lugens). Phytoparasitica 35:346–356
Arora AK, Douglas AE (2017) Hype or opportunity? Using microbial symbionts in novel strategies for insect pest control. J Insect Physiol 103:10–17
Briones-Roblero CI, Rodríguez-Díaz R, Santiago-Cruz JA, Zúñiga G, Rivera-Orduña FN (2017) Degradation capacities of bacteria and yeasts isolated from the gut of Dendroctonus rhizophagus (Curculionidae: Scolytinae). Folia Microbiol (Praha) 62:1–9
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Cheng X, Zhu L, He G (2013) Towards understanding of molecular interactions between rice and the brown planthopper. Mol Plant 6:621–634
Cohen MB, Alam SN, Medina EB, Bernal CC (2010) Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: mechanism and role in successful N. lugens management in Central Luzon. Philippines Entomol Exp Appl 85:221–229
Crotti E, Balloi A, Hamdi C, Sansonno L, Marzorati M, Gonella E, Favia G, Cherif A, Bandi C, Alma A, Daffonchio D (2012) Microbial symbionts: a resource for the management of insect-related problems. Microb Biotechnol 5:307–317
De Vooght L, Caljon G, De Ridder K, Van Den Abbeele J (2014) Delivery of a functional anti-trypanosome Nanobody in different tsetse fly tissues via a bacterial symbiont Sodalis glossinidius. Microb Cell Fact 13:156
Dong SZ, Pang K, Bai X, Yu XP, Hao PY (2011) Identification of two species of yeast-like symbiotes in the brown planthopper, Nilaparvata lugens. Curr Microbiol 62:1133–1138
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34
Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA (2016) Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 9:771–774
Engel P, Moran NA (2013) The gut microbiota of insects: diversity in structure and function. FEMS Microbiol Rev 37:699–735
Fan HW, Noda H, Xie HQ, Suetsugu Y, Zhu QH, Zhang CX (2015) Genomic analysis of an ascomycete fungus from the rice planthopper reveals how it adapts to an endosymbiotic lifestyle. Genome Biol Evol 7:2623–2634
Frago E, Dicke M, Godfray HC (2012) Insect symbionts as hidden players in insect-plant interactions. Trends Ecol Evol 27:705–711
Gauthier JP, Outreman Y, Mieuzet L, Simon JC (2015) Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA. PLoS ONE 10:e0120664
Hammer TJ, Bowers MD (2015) Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia 179:1–14
Hemingway J, Karunaratne SHPP, Claridge MF (1999) Insecticide resistance spectrum and underlying resistance mechanisms in tropical populations of the brown planthopper (Nilaparvata lugens) collected from rice and the wild grass Leersia hexandra. Int J Pest Manage 45:215–223
Hou Y, Ma Z, Dong S, Chen YH, Yu X (2013) Analysis of yeast-like symbiote diversity in the brown planthopper (BPH), Nilaparvata lugens Stål, using a novel nested PCR-DGGE protocol. Curr Microbiol 67:263–270
Jena KK, Kim SM (2010) Current status of brown planthopper (BPH) resistance and genetics. Rice 3:161–171
Landry M, Comeau AM, Derome N, Cusson M, Levesque RC (2015) Composition of the spruce budworm (Choristoneura fumiferana) midgut microbiota as affected by rearing conditions. PLoS ONE 10:e0144077
Louca S, Parfrey LW, Doebeli M (2016) Decoupling function and taxonomy in the global ocean microbiome. Science 353:1272–1277
Malacrinò A, Campolo O, Medina RF, Palmeri V (2018) Instar- and host-associated differentiation of bacterial communities in the Mediterranean fruit fly Ceratitis capitata. PLoS ONE 13:e0194131
Mason CJ, Campbell AM, Scully ED, Hoover K (2019) Bacterial and fungal midgut community dynamics and transfer between mother and brood in the Asian longhorned beetle (Anoplophora glabripennis), an invasive Xylophage. Microb Ecol 77:230–242
McLean AH, van Asch M, Ferrari J, Godfray HC (2011) Effects of bacterial secondary symbionts on host plant use in pea aphids. Proc Biol Sci 278:760–766
Montllor CB, Maxmen A, Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol 27:189–195
Noda H, Omura T (1992) Purification of yeast-like symbiotes of planthoppers. J Invertebr Pathol 59:104–105
Ohkuma M, Noda S, Hongoh Y, Kudo T (2002) Diverse bacteria related to the bacteroides subgroup of the CFB phylum within the gut symbiotic communities of various termites. Biosci Biotechnol Biochem 66:78–84
Ojha A, Zhang WA (2019) Comparative study of microbial community and dynamics of Asaia in the brown planthopper from susceptible and resistant rice varieties. BMC Microbiol 19:139
Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci 100:1803–1807
Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266
Pang K, Dong SZ, Hou Y, Bian YL, Yang K, Yu XP (2012) Cultivation, identification and quantification of one species of yeast-like symbiotes, Candida, in the rice brown planthopper, Nilaparvata lugens. Insect Sci 19:477–484
Paulson JN, Pop M, Bravo HC (2012) Metastats: an improved statistical method for analysis of metagenomic data. Genome Biol 12:P17
Renoz F, Pons I, Vanderpoorten A, Bataille G, Noël C, Foray V, Pierson V, Hance T (2019) Evidence for gut-associated Serratia symbiotica in wild aphids and ants provides new perspectives on the evolution of bacterial mutualism in insects. Microb Ecol 78:159–169
Schloss PD, Delalibera I, Handelsman J, Raffa KF (2006) Bacteria associated with the guts of two wood-boring beetles: Anoplophora glabripennis and Saperda vestita (Cerambycidae). Environ Entomol 35:625–629
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Su MM, Guo L, Tao YL, Zhang YJ, Wan FH, Chu D (2016) Effects of host plant factors on the bacterial communities associated with two whitefly sibling species. PLoS ONE 11:e0152183
Tang M, Lu L, Jing S, Zhu L, He G (2010) Bacterial symbionts of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Appl Environ Microbiol 76:1740–1745
van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, Higgs S, O'Neill SL (2012) Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis 6:e1892
Vijayakumar MM, More RP, Rangasamy A, Gandhi GR, Muthugounder M, Thiruvengadam V, Samaddar S, Jalali SK, Sa T (2018) Gut Bacterial diversity of insecticide-susceptible and -resistant nymphs of the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae) and elucidation of their putative functional roles. J Microbiol Biotechnol 28:976–986
Wang Y, Chen J, Zhu YC, Ma C, Huang Y, Shen J (2008) Susceptibility to neonicotinoids and risk of resistance development in the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Pest Manag Sci 64:1278–1284
Wang WX, Zhu TH, Lai FX, Fu Q (2015) Diversity and infection frequency of symbiotic bacteria in different populations of the rice brown planthopper in China. J Entomol Sci 50:47–66
Wang ZL, Wang TZ, Zhu HF, Pan HB, Yu XP (2019) Diversity and dynamics of microbial communities in brown planthopper at different developmental stages revealed by high-throughput amplicon sequencing. Insect Sci. https://doi.org/10.1111/1744-7917.12729
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751
Xiao X, Gurr GM, Vasseur L, Zheng D, Zhong H, Qin B, Lin J, Wang Y, Song F, Li Y, Lin H, You M (2017) Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front Microbiol 8:663
Xu HX, Zheng XS, Yang YJ, Wang X, Ye GY, Lu ZX (2014) Bacterial community in different populations of rice brown planthopper Nilaparvata lugens (Stål). Rice Sci 21:59–64
Yu H, Ji R, Ye W, Chen H, Lai W, Fu Q, Lou Y (2014) Transcriptome analysis of fat bodies from two brown planthopper (Nilaparvata lugens) populations with different virulence levels in rice. PLoS ONE 9:e88528
Yun JH, Roh SW, Whon TW, Jung MJ, Kim MS, Park DS, Yoon C, Nam YD, Kim YJ, Choi JH, Kim JY, Shin NR, Kim SH, Lee WJ, Bae JW (2014) Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Environ Microbiol 80:5254–5264
Zhang JH, Yu N, Xu XX, Liu ZW (2018a) Community structure, dispersal ability and functional profiling of microbiome existing in fat body and ovary of the brown planthopper, Nilaparvata lugens. Insect Sci 26:683–694
Zhang Y, Tang T, Li W, Cai T, Li J, Wan H (2018b) Functional profiling of the gut microbiomes in two different populations of the brown planthopper, Nilaparvata lugens. J Asia-Pac Entomol 21:1309–1314
Zhao Y, Zhang S, Luo JY, Wang CY, Lv LM, Cui JJ (2016) Bacterial communities of the cotton aphid Aphis gossypii associated with Bt cotton in northern China. Sci Rep 6:22958
Zhao X, Zhang X, Chen Z, Wang Z, Lu Y, Cheng D (2018) The divergence in bacterial components associated with Bactrocera dorsalis across developmental stages. Front Microbiol 9:114
Acknowledgements
This work was financially supported by the Natural Science Foundation of Zhejiang Province (Grant No. LR19C140001), the National Natural Science Foundation of China (Grant no. 31972347; 31601698) and the Key Research and Development Program of Zhejiang Province (Grant No. 2019C02015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
203_2020_2013_MOESM1_ESM.rar
Supplementary file1 (RAR 283 kb) Table S1 Shared and unique bacterial OTUs in the gut of two BPH populations rearing on a susceptible rice variety (TN1) and a resistance rice variety (ASD7), respectively.Table S2 Function classification of bacterial community in the gut of BPH rearing on a susceptible rice variety (TN1) and a resistance rice variety (ASD7) by FAPROTAX.Fig. S1. Rarefaction curves of bacterial 16S rRNA sequences in BPH rearing on a susceptible rice variety (TN1) and a resistance rice variety (ASD7).
Rights and permissions
About this article
Cite this article
Wang, ZL., Pan, Hb., Wu, W. et al. The gut bacterial flora associated with brown planthopper is affected by host rice varieties. Arch Microbiol 203, 325–333 (2021). https://doi.org/10.1007/s00203-020-02013-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02013-8