Abstract
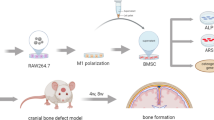
The potential risk associated with ACP nanoparticles (ACP NPs) cultured with immune cells and their indirect effects on osteogenesis have not been studied deeply. This project aims to evaluate the safety of ACP NPs in macrophages, the responses of macrophages (macrophage polarization, the cytokine secretion pattern of macrophages and intracellular homeostasis) to ACP NPs and the effect of ACP NPs/macrophage-modulated environments on the osteogenic ability of BMSCs. The cell proliferation rate and apoptosis were detected by CCK-8 and Annexin V Apoptosis Detection kits. ROS and autophagy expression were evaluated by ROS test kits and Western blot (WB). Macrophage polarization and cytokine expression were determined by SEM, cytoskeletal staining, RT-PCR and ELISA. TMT™ quantitative protein analysis was used to evaluate protein expression. BMSC osteogenic differentiation was detected by ALP staining, Alizarin Red solution staining and RT-PCR. ACP NPs were safe to macrophages but promoted autophagy and induced ROS production at high concentrations. ACP NPs changed morphology of macrophages and induced polarization into M1 type, thus promoting the expression of inflammatory cytokines. ACP NPs/macrophage-modulated environments weakened the osteogenic ability of BMSCs. ACP NPs polarize macrophages into the M1 phenotype and change the cytokine secretion pattern. ACP NPs/macrophage-modulated environments weaken the osteogenic ability of BMSCs. ACP NPs may cause aseptic inflammation and attenuate osteogenesis.










Similar content being viewed by others
References
Choi, H., N.J. Park, O. Jamiyandorj, M.H. Hong, S. Oh, Y.B. Park, and S. Kim. 2012. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with synthetic cell binding peptide sequences. Journal of Periodontal & Implant Science 42 (5): 166–172.
Velard, F., J. Braux, J. Amedee, and P. Laquerriere. 2013. Inflammatory cell response to calcium phosphate biomaterial particles: An overview. Acta Biomaterialia 9 (2): 4956–4963.
Accorsi-Mendonça, T., M.B. Conz, T.C. Barros, L.A. de Sena, A. Soares Gde, and J.M. Granjeiro. 2008. Physicochemical characterization of two deproteinized bovine xenografts. Brazilian Oral Research 22 (1): 5–10.
Daculsi, G., and J.P. Legeros. 1996. Three-dimensional defects in hydroxyapatite of biological interest. Journal of Biomedical Materials Research 31 (4): 495–501.
Prudhommeaux, F., C. Schiltz, F. Lioté, A. Hina, R. Champy, B. Bucki, E. Ortiz-Bravo, A. Meunier, C. Rey, and T. Bardin. 1996. Variation in the inflammatory properties of basic calcium phosphate crystals according to crystal type. Arthritis and Rheumatism 39 (8): 1319–1326.
Wang, J., D. Liu, B. Guo, X. Yang, X. Chen, and X. Zhu. 2017. fan Y, Zhang X. Role of biphasic calcium phosphate ceramic-mediated secretion of signaling molecules by macrophages in migration and osteoblastic differentiation of MSCs. Acta Biomaterialia 51: 447–460.
Jones, J.A., D.T. Chang, H. Meyerson, E. Colton, I.K. Kwon, T. Matsuda, and J.M. Anderson. 2007. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. Journal of Biomedical Materials Research. Part A 83 (3): 585–596.
Oh, J., A.E. Riek, S. Weng, M. Petty, D. Kim, M. Colonna, M. Cella, and C. Bernal-Mizrachi. 2012. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. The Journal of Biological Chemistry 287 (15): 11629–11641.
Rao, A.J., E. Gibon, T. Ma, Z. Yao, R.L. Smith, and S.B. Goodman. 2012. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomaterialia 8 (7): 2815–2823.
Lotsari, A., A.K. Rajasekharan, M. Halvarsson, and M. Andersson. 2018. Transformation of amorphous calcium phosphate to bone-like apatite. Nature Communications 9 (1): 4170.
Iafisco, M., L. Degli Esposti, G.B. Ramírez-Rodríguez, F. Carella, J. Gómez-Morales, A.C. Ionescu, E. Brambilla, A. Tampieri, and J.M. Delgado-López. 2018. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Scientific Reports 8 (1): 17016.
Cai, X., B. Han, Y. Liu, F. Tian, F. Liang, and X. Wang. 2017. Chlorhexidine-loaded amorphous calcium phosphate nanoparticles for inhibiting degradation and inducing mineralization of type I collagen. ACS Applied Materials & Interfaces 9 (15): 12949–12958.
Allegrini, Sergio, Jr., Antonio Carlos da Silva, Maristela Tsujita, Marcos Barbosa Salles, Sergio Alexandre Gehrke, and Francisco José Correa Braga. 2018. Amorphous calcium phosphate (ACP) in tissue repair process.81 (6): 579–589.
Edwards, Felicity C., Amir Taheri, Sophie C. Dann, and Julian F. Dye. 2011. Characterization of cytolytic neutrophil activation in vitro by amorphous hydrated calcium phosphate as a model of biomaterial inflammation. Journal of Biomedical Materials Research. Part A 96 (3): 552–565.
Feng, G., C. Qin, X. Yi, J. Xia, J. Chen, X. Chen, T. Chen, and X. Jiang. 2018. Effect of novel bioresorbable scaffold composed of poly-L-lactic acid and amorphous calcium phosphate nanoparticles on inflammation and calcification of surrounding tissues after implantation. Journal of Materials Science. Materials in Medicine 29 (8): 112.
Klopfleisch, R. 2016. Macrophage reaction against biomaterials in the mouse model - Phenotypes, functions and markers. Acta Biomaterialia 43: 3–13.
Brancato, S.K., and J.E. Albina. 2011. Wound macrophages as key regulators of repair: Origin, phenotype, and function. Am J Pathol 178 (1): 19–25.
Chen, S., J.A. Jones, Y. Xu, H.Y. Low, J.M. Anderson, and K.W. Leong. 2010. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials. 31 (13): 3479–3491.
Lee, H.S., S.J. Stachelek, N. Tomczyk, M.J. Finley, R.J. Composto, and D.M. Eckmann. 2013. Correlating macrophage morphology and cytokine production resulting from biomaterial contact. Journal of Biomedical Materials Research. Part A 101 (1): 203–212.
Manke, A., L. Wang, and Y. Rojanasakul. 2013. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Research International 2013: 942916.
Gordon, P.B., I. Holen, M. Fosse, J.S. Røtnes, and P.O. Seglen. 1993. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. The Journal of Biological Chemistry 268 (35): 26107–26112.
Decuypere, J.P., G. Bultynck, and J.B. Parys. 2011. A dual role for Ca(2+) in autophagy regulation. Cell Calcium 50 (3): 242–250.
Cárdenas, C., R.A. Miller, I. Smith, T. Bui, J. Molgó, M. Müller, H. Vais, K.H. Cheung, J. Yang, I. Parker, C.B. Thompson, M.J. Birnbaum, K.R. Hallows, and J.K. Foskett. 2010. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 142 (2): 270–283.
Williams, A., S. Sarkar, P. Cuddon, E.K. Ttofi, S. Saiki, F.H. Siddiqi, L. Jahreiss, A. Fleming, D. Pask, P. Goldsmith, C.J. O’Kane, R.A. Floto, and D.C. Rubinsztein. 2008. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nature Chemical Biology 4 (5): 295–305.
Høyer-Hansen, M., L. Bastholm, P. Szyniarowski, M. Campanella, G. Szabadkai, T. Farkas, K. Bianchi, N. Fehrenbacher, F. Elling, R. Rizzuto, I.S. Mathiasen, and M. Jäättelä. 2007. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 25 (2): 193–205.
Chen, Z., S. Ni, S. Han, R. Crawford, S. Lu, F. Wei, J. Chang, C. Wu, and Y. Xiao. 2017. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale. 9 (2): 706–718.
Murray, P.J., J.E. Allen, S.K. Biswas, E.A. Fisher, D.W. Gilroy, S. Goerdt, S. Gordon, J.A. Hamilton, L.B. Ivashkiv, T. Lawrence, M. Locati, A. Mantovani, F.O. Martinez, J.L. Mege, D.M. Mosser, G. Natoli, J.P. Saeij, J.L. Schultze, K.A. Shirey, A. Sica, J. Suttles, I. Udalova, J.A. van Ginderachter, S.N. Vogel, and T.A. Wynn. 2014. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 41 (1): 14–20.
Bain, C.C., and A. Schridde. 2733. Origin, differentiation, and function of intestinal macrophages. Frontiers in Immunology 2018 (27): 9.
Murray, P.J. 2017. Macrophage Polarization. Annual Review of Physiology 79: 541–566.
Hotchkiss, K.M., G.B. Reddy, S.L. Hyzy, Z. Schwartz, B.D. Boyan, and R. Olivares-Navarrete. 2016. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomaterialia 31: 425–434.
Mantovani, A., S.K. Biswas, M.R. Galdiero, A. Sica, and M. Locati. 2013. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of Pathology 229 (2): 176–185.
Pajarinen, J., V.P. Kouri, E. Jämsen, T.F. Li, J. Mandelin, and Y.T. Konttinen. 2013. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomaterialia 9 (11): 9229–9240.
Miao, X., X. Leng, Q. Zhang, et al. 2017. Int J Mol Sci 18 (2).
Shi, M., Z. Chen, S. Farnaghi, T. Friis, X. Mao, Y. Xiao, and C. Wu. 2016. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomaterialia 30: 334–344.
Lucarelli, M., A.M. Gatti, G. Savarino, P. Quattroni, L. Martinelli, E. Monari, and D. Boraschi. 2004. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. European Cytokine Network 15 (4): 339–346.
Yen, H.J., S.H. Hsu, and C.L. Tsai. 2009. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 5 (13): 1553–1561.
Nishanth, R.P., R.G. Jyotsna, J.J. Schlager, S.M. Hussain, and P. Reddanna. 2011. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFκB signaling pathway. Nanotoxicology. 5 (4): 502–516.
Ma, Q.L., L.Z. Zhao, R.R. Liu, B.Q. Jin, W. Song, Y. Wang, Y.S. Zhang, L.H. Chen, and Y.M. Zhang. 2014. Improved implant osseointegration of a nanostructured titanium surface viamediation of macrophage polarization. Biomaterials. 35 (37): 9853–9867.
Chen, Z., A. Bachhuka, S. Han, F. Wei, S. Lu, R.M. Visalakshan, K. Vasilev, and Y. Xiao. 2017. Tuning chemistry and topography of nanoengineered surfaces to manipulateimmune response for bone regeneration applications. ACS Nano 11 (5): 4494–4506.
Yang, C.L., Y.H. Sun, W.H. Yu, X.Z. yin, J. Weng, and B. Feng. 2018. Modulation of macrophage phenotype through controlled release of interleukin-4 from gelatine coatings on titanium surfaces. European Cells & Materials 36: 15–29.
Jamalpoor, Z., A. Asgari, M.H. Lashkari, A. Mirshafiey, and M. Mohsenzadegan. 2018. Modulation of macrophage polarization for bone tissue engineering applications. Iranian Journal of Allergy, Asthma, and Immunology 17 (5): 398–408.
Zhang, Y., T. Böse, R.E. Unger, J.A. Jansen, C.J. Kirkpatrick, and J.J.J.P. van den Beucken. 2017. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell and Tissue Research 369 (2): 273–286.
Zhang, Z., Y. Xie, H. Pan, L. Huang, and X. Zheng. 2018. Influence of patterned titanium coatings on polarization of macrophage and osteogenic differentiation of bone marrow stem cells. Journal of Biomaterials Applications 32 (7): 977–986.
Park, H.C., H. Quan, T. Zhu, Y. Kim, B. Kim, and H.C. Yang. 2017. The effects of M1 and M2 macrophages on odontogenic differentiation of human dental pulp cells. Journal of Endodontia 43 (4): 596–601.
Chudinova, E.A., M.A. Surmeneva, A.S. Timin, T.E. Karpov, A. Wittmar, M. Ulbricht, A. Ivanova, K. Loza, O. Prymak, A. Koptyug, M. Epple, and R.A. Surmenev. 2018. Adhesion, proliferation, and osteogenic differentiation of human mesenchymal stem cells on additively manufactured Ti6Al4V alloy scaffolds modified with calcium phosphate nanoparticles. Colloids and Surfaces. B, Biointerfaces 176: 130–139.
Zhao, F., B. Lei, X. Li, Y. Mo, R. Wang, D. Chen, and X. Chen. 2018. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials. 178: 36–47.
Zhang, W., F. Zhao, D. Huang, X. Fu, X. Li, and X. Chen. 2016. Strontium-substituted submicrometer bioactive glasses modulate macrophage responses for improved bone regeneration. ACS Applied Materials & Interfaces 8 (45): 30747–30758.
Funding
This work was supported financially by the National Natural Science Fund (81600904 and 81771102), the High Technology Research and Development Program of China (863 program, 2015AA033502) and the incubation programme of Guangzhou Medical University (No. B185004177).
Author information
Authors and Affiliations
Contributions
LJC, HCL and LQS designed the research. LJC was responsible for characterizing ACP NPs and detecting macrophage polarization and cytokine expression. ROS and autophagy expression were evaluated by LJC and PYQ. TMT™ quantitative protein analysis and BMSC osteogenic differentiation were detected by LJC and PYQ. LJC generated the figures and drafted the manuscript, which was critically revised by HCL and LQS. All authors read, corrected and approved the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 4451 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Qiao, P., Liu, H. et al. Amorphous Calcium Phosphate NPs Mediate the Macrophage Response and Modulate BMSC Osteogenesis. Inflammation 44, 278–296 (2021). https://doi.org/10.1007/s10753-020-01331-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01331-9