Abstract
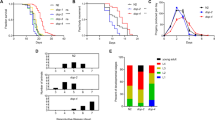
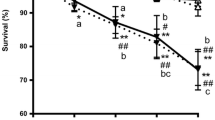
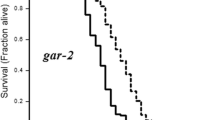
Haloperidol is a typical antipsychotic drug commonly used to treat a broad range of psychiatric disorders related to dysregulations in the neurotransmitter dopamine (DA). DA modulates important physiologic functions and perturbations in Caenorhabditis elegans (C. elegans) and, its signaling have been associated with alterations in behavioral, molecular, and morphologic properties in C. elegans. Here, we evaluated the possible involvement of dopaminergic receptors in the onset of these alterations followed by haloperidol exposure. Haloperidol increased lifespan and decreased locomotor behavior (basal slowing response, BSR, and locomotion speed via forward speed) of the worms. Moreover, locomotion speed recovered to basal conditions upon haloperidol withdrawal. Haloperidol also decreased DA levels, but it did not alter neither dop-1, dop-2, and dop-3 gene expression, nor CEP dopaminergic neurons’ morphology. These effects are likely due to haloperidol’s antagonism of the D2-type DA receptor, dop-3. Furthermore, this antagonism appears to affect mechanistic pathways involved in the modulation and signaling of neurotransmitters such as octopamine, acetylcholine, and GABA, which may underlie at least in part haloperidol’s effects. These pathways are conserved in vertebrates and have been implicated in a range of disorders. Our novel findings demonstrate that the dop-3 receptor plays an important role in the effects of haloperidol.








Similar content being viewed by others
References
Engleman EA, Katner SN, Neal-Beliveau BS (2016) Caenorhabditis elegans as a model to study the molecular and genetic mechanisms of drug addiction. In: Progress in Molecular Biology and Translational Science
de Bono M, Villu Maricq A (2005) Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. https://doi.org/10.1146/annurev.neuro.27.070203.144259
ER Sawin, R Ranganathan, HR Horvitz (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron
Nass R, Hall DH, Miller DM, Blakely RD (2002) Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99:3264–3269. https://doi.org/10.1073/pnas.042497999
Hansen FH, Skjørringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, Andreassen TF, Holy M et al (2014) Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J Clin Invest 124:3107–3120. https://doi.org/10.1172/JCI73778
Solanto MV (2002) Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. In: Behavioural Brain Research
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Hornykiewicz O (2006) The discovery of dopamine deficiency in the parkinsonian brain. In: Journal of Neural Transmission, Supplement
Mersha M, Formisano R, McDonald R et al (2013) GPA-14, a Gαi subunit mediates dopaminergic behavioral plasticity in C. elegans. Behav Brain Funct. https://doi.org/10.1186/1744-9081-9-16
Hills T, Brockie PJ, Maricq AV (2004) Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci 24:1217–1225. https://doi.org/10.1523/JNEUROSCI.1569-03.2004
Zhen M, Samuel ADT (2015) C. elegans locomotion: small circuits, complex functions. Curr Opin Neurobiol
Kang L, Gao J, Schafer WR, Xie Z, Xu XZS (2010) C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron 67:381-391. https://doi.org/10.1016/j.neuron.2010.06.032
(2008) WormAtlas. Choice Rev Online. https://doi.org/10.5860/choice.46-0292
Maeder CI, Kim JI, Liang X, Kaganovsky K, Shen A, Li Q, Li Z, Wang S et al (2018) The THO complex coordinates transcripts for synapse development and dopamine neuron survival. Cell. 174:1436–1449.e20. https://doi.org/10.1016/j.cell.2018.07.046
Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI (2013) Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154:1023–1035. https://doi.org/10.1016/j.cell.2013.08.001
Fujiwara M, Sengupta P, McIntire SL (2002) Regulation of body size and behavioral state of C. elegans by sensory perception and the egl-4 cGMP-dependent protein kinase. Neuron. https://doi.org/10.1016/S0896-6273(02)01093-0
Suo S, Sasagawa N, Ishiura S (2003) Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem 86:869–878. https://doi.org/10.1046/j.1471-4159.2003.01896.x
Chase DL, Koelle MR (2007) Biogenic amine neurotransmitters in C. elegans. WormBook
Pandey P, Mersha MD, Dhillon HS (2013) A synergistic approach towards understanding the functional significance of dopamine receptor interactions. J Mol Signal 8:1-8. https://doi.org/10.1186/1750-2187-8-13
Sanyal S, Wintle RF, Kindt KS, , Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hébert TE, van der Kooy D, Schafer WR, Culotti JG, van Tol HHM (2004) Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J 23:473-482. https://doi.org/10.1038/sj.emboj.7600057
Chase DL, Pepper JS, Koelle MR (2004) Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci 7:1096–1103. https://doi.org/10.1038/nn1316
Suo S, Culotti JG, Van Tol HHM (2009) Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J. https://doi.org/10.1038/emboj.2009.194
Kindt KS, Quast KB, Giles AC et al (2007) Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. https://doi.org/10.1016/j.neuron.2007.07.023
Ezak MJ, Ferkey DM (2010) The C. elegans D2-like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS One. https://doi.org/10.1371/journal.pone.0009487
Ezcurra M, Tanizawa Y, Swoboda P, Schafer WR (2011) Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J. https://doi.org/10.1038/emboj.2011.22
Chase DL, Koelle MR (2004) Genetic analysis of RGS protein function in Caenorhabditis elegans. Methods Enzymol. https://doi.org/10.1016/S0076-6879(04)89018-9
Allen AT, Maher KN, Wani KA, Betts KE, Chase DL (2011) Coexpressed D1- and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans. Genetics. 188:579–590. https://doi.org/10.1534/genetics.111.128512
Reckziegel P, Chen P, Caito S, Gubert P, Soares FAA, Fachinetto R, Aschner M (2016) Extracellular dopamine and alterations on dopamine transporter are related to reserpine toxicity in Caenorhabditis elegans. Arch Toxicol 90:633–645. https://doi.org/10.1007/s00204-015-1451-7
Refai O, Blakely RD (2019) Blockade and reversal of swimming-induced paralysis in C. elegans by the antipsychotic and D2-type dopamine receptor antagonist azaperone. Neurochem Int. https://doi.org/10.1016/j.neuint.2018.05.013
Omura DT, Clark DA, Samuel ADT, Horvitz HR (2012) Dopamine signaling is essential for precise rates of locomotion by C. elegans. PLoS One. https://doi.org/10.1371/journal.pone.0038649
Akinyemi AJ, Miah MR, Ijomone OM, Tsatsakis A, Soares FAA, Tinkov AA, Skalny AV, Venkataramani V et al (2019) Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: involvement of the dopamine transporter. Toxicol Reports 6:833–840. https://doi.org/10.1016/j.toxrep.2019.08.001
Chen P, DeWitt MR, Bornhorst J et al (2015) Age- and manganese-dependent modulation of dopaminergic phenotypes in a C. elegans DJ-1 genetic model of Parkinson’s disease. Metallomics. https://doi.org/10.1039/c4mt00292j
Wang D, Yu Y, Li Y et al (2014) Dopamine receptors antagonistically regulate behavioral choice between conflicting alternatives in C. elegans e115985. PLoS One. https://doi.org/10.1371/journal.pone.0115985
Manalo RVM, Medina PMB (2018) Caffeine protects dopaminergic neurons from dopamine-induced neurodegeneration via synergistic adenosine-dopamine D2-like receptor interactions in transgenic Caenorhabditis elegans. Front Neurosci 12. https://doi.org/10.3389/fnins.2018.00137
Dagenhardt J, Trinh A, Sumner H, Scott J, Aamodt E, Dwyer DS (2017) Insulin signaling deficiency produces immobility in Caenorhabditis elegans that models diminished motivation states in man and responds to antidepressants. Mol Neuropsychiatry 3:97–107. https://doi.org/10.1159/000478049
Monte GG, Nani JV, de Almeida Campos MR, Dal Mas C, Marins LAN, Martins LG, Tasic L, Mori MA et al (2019) Impact of nuclear distribution element genes in the typical and atypical antipsychotics effects on nematode Caenorhabditis elegans: putative animal model for studying the pathways correlated to schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry 92:19–30. https://doi.org/10.1016/j.pnpbp.2018.12.010
Karmacharya R, Lynn SK, Demarco S et al (2011) Behavioral effects of clozapine: Involvement of trace amine pathways in C. elegans and M. musculus. Brain Res. https://doi.org/10.1016/j.brainres.2011.04.010
Dwyer SD, Poonam A, Rashmi T, et al (2015) Social feeding in Caenorhabditis elegans is modulated by antipsychotic drugs and calmodulin and may serve as a protophenotype for asociality. Neuropharmacology
Donohoe DR, Weeks K, Aamodt EJ, Dwyer DS (2008) Antipsychotic drugs alter neuronal development including ALM neuroblast migration and PLM axonal outgrowth in Caenorhabditis elegans. Int J Dev Neurosci 26:371-380. https://doi.org/10.1016/j.ijdevneu.2007.08.021
Donohoe DR, Aamodt EJ, Osborn E, Dwyer DS (2006) Antipsychotic drugs disrupt normal development in Caenorhabditis elegans via additional mechanisms besides dopamine and serotonin receptors. Pharmacol Res 54:361–372. https://doi.org/10.1016/j.phrs.2006.07.002
McCormick AV, Wheeler JM, Guthrie CR, Liachko NF, Kraemer BC (2013) Dopamine D2 receptor antagonism suppresses tau aggregation and neurotoxicity. Biol Psychiatry 73:464–471. https://doi.org/10.1016/j.biopsych.2012.08.027
Andreassen OA, Jørgensen HA (2000) Neurotoxicity associated with neuroleptic-induced oral dyskinesias in rats: implications for tardive dyskinesia? Prog Neurobiol 61:525–541
Keilhoff G, Grecksch G, Bernstein HG, Roskoden T, Becker A (2010) Risperidone and haloperidol promote survival of stem cells in the rat hippocampus. Eur Arch Psychiatry Clin Neurosci 260:151–162. https://doi.org/10.1007/s00406-009-0033-1
Tran PV, Dellva MA, Tollefson GD, Beasley CM Jr, Potvin JH, Kiesler GM (1997) Extrapyramidal symptoms and tolerability of olanzapine versus haloperidol in the acute treatment of schizophrenia. J Clin Psychiatry 58:205–211. https://doi.org/10.4088/JCP.v58n0505
Nishigaki A, Kawano T, Iwata H, Aoyama B, Yamanaka D, Tateiwa H, Shigematsu-Locatelli M, Eguchi S et al (2019) Acute and long-term effects of haloperidol on surgery-induced neuroinflammation and cognitive deficits in aged rats. J Anesth 33:416–425. https://doi.org/10.1007/s00540-019-02646-0
Guzen FP, Cavalcanti JRL d P, Cavalcanti DML d P et al (2019) Haloperidol-induced preclinical tardive dyskinesia model in rats. Curr Protoc Neurosci 88:e68. https://doi.org/10.1002/cpns.68
Fachinetto R, Villarinho JG, Wagner C, Pereira RP, Ávila DS, Burger ME, Calixto JB, Rocha JBT et al (2007) Valeriana officinalis does not alter the orofacial dyskinesia induced by haloperidol in rats: role of dopamine transporter. Prog Neuro-Psychopharmacology Biol Psychiatry 31:1478–1486. https://doi.org/10.1016/j.pnpbp.2007.06.028
Park SH, Song YS, Moon BS, Lee BC, Park HS, Kim SE (2019) Combination of in vivo [123I]FP-CIT SPECT and microdialysis reveals an antipsychotic drug haloperidol-induced synaptic dopamine availability in the rat midbrain and striatum. Exp Neurobiol 28:602–611. https://doi.org/10.5607/en.2019.28.5.602
Röpke J, Busanello A, Leal CQ, de Moraes Reis E, de Freitas CM, Villarinho JG, Figueira FH, Mello CF et al (2014) Anandamide attenuates haloperidol-induced vacuous chewing movements in rats. Prog Neuro-Psychopharmacology Biol Psychiatry. 54:195–199. https://doi.org/10.1016/j.pnpbp.2014.04.006
Ceretta APC, de Freitas CM, Schaffer LF, Reinheimer JB, Dotto MM, de Moraes Reis E, Scussel R, Machado-de-Ávila RA et al (2018) Gabapentin reduces haloperidol-induced vacuous chewing movements in mice. Pharmacol Biochem Behav 166:21–26. https://doi.org/10.1016/j.pbb.2018.01.003
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics
Benedetto A, Au C, Avila DS, Milatovic D, Aschner M (2010) Extracellular dopamine potentiates Mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3- dependent manner in Caenorhabditis elegans. PLoS Genet 6:e1001084. https://doi.org/10.1371/journal.pgen.1001084
Wong JMT, Malec PA, Mabrouk OS, Ro J, Dus M, Kennedy RT (2016) Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J Chromatogr A 1446:78–90. https://doi.org/10.1016/j.chroma.2016.04.006
Yohn SE, Foster DJ, Covey DP, Moehle MS, Galbraith J, Garcia-Barrantes PM, Cho HP, Bubser M et al (2018) Activation of the mGlu1 metabotropic glutamate receptor has antipsychotic-like effects and is required for efficacy of M4 muscarinic receptor allosteric modulators. Mol Psychiatry. https://doi.org/10.1038/s41380-018-0206-2
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 25:402–408. https://doi.org/10.1006/meth.2001.1262
Sulston J, Dew M, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163:215–226. https://doi.org/10.1002/cne.901630207
Maulik M, Mitra S, Bult-Ito A, et al (2017) Behavioral phenotyping and pathological indicators of Parkinson’s disease in C. elegans models. Front Genet
Bornhorst J, Chakraborty S, Meyer S, Lohren H, Brinkhaus SG, Knight AL, Caldwell KA, Karst U, Schwerdtle T, Bowman A, Aschner M (2014) The effects of pdr1, djr1.1 and pink1 loss in manganese-induced toxicity and the role of alphasynuclein in C. elegans. Metallomics 6:476-490. https://doi.org/10.1039/c3mt00325f
Oteri A, Mazzaglia G, Pecchioli S, , Molokhia M, Ulrichsen SP, Pedersen L, Poluzzi E, de Ponti F, Garbe E, Schink T, Herings R, Bezemer ID, Sturkenboom MCJM, Trifirò G (2016) Prescribing pattern of antipsychotic drugs during the years 1996–2010: a population-based database study in Europe with a focus on torsadogenic drugs. Br J Clin Pharmacol 82:487-497. https://doi.org/10.1111/bcp.12955
Hálfdánarson Ó, Zoëga H, Aagaard L, Bernardo M, Brandt L, Fusté AC, Furu K, Garuoliené K et al (2017) International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur Neuropsychopharmacol 27:1064–1076. https://doi.org/10.1016/j.euroneuro.2017.07.001
Hao L, Buttner EA (2014) Methods for studying the mechanisms of action of antipsychotic drugs in Caenorhabditis elegans. J Vis Exp. https://doi.org/10.3791/50864
Wang Q, Timberlake MA, Prall K, Dwivedi Y (2017) The recent progress in animal models of depression. Prog. Neuro-Psychopharmacology Biol Psychiatry
Osuna-Luque J, Rodríguez-Ramos Á, Gámez-del-Estal M del M, Ruiz-Rubio M (2018) Behavioral Mechanisms That Depend on Dopamine and Serotonin in Caenorhabditis elegans Interact With the Antipsychotics Risperidone and Aripiprazole. J Exp Neurosci 12:117906951879862. https://doi.org/10.1177/1179069518798628
Mocko JB, Kern A, Moosmann B, Behl C, Hajieva P (2010) Phenothiazines interfere with dopaminergic neurodegeneration in Caenorhabditis elegans models of Parkinson’s disease. Neurobiol Dis 40:120–129. https://doi.org/10.1016/j.nbd.2010.03.019
Smith ED, Kaeberlein TL, Lydum BT, Sager J, Welton KL, Kennedy BK, Kaeberlein M (2008) Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev Biol 8. https://doi.org/10.1186/1750-2187-8-13
Suo S, Culotti JG, Van Tol HHM (2009) Dopamine suppresses octopamine signaling in C. elegans: possible involvement of dopamine in the regulation of lifespan. Aging (Albany NY). https://doi.org/10.18632/aging.100097
Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. https://doi.org/10.1016/S0896-6273(00)81199-X
Gjorgjieva J, Biron D, Haspel G (2014) Neurobiology of caenorhabditis elegans locomotion: where do we stand? Bioscience
White JG, Southgate E, Thomson JN, Brenner S (1976) The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond Ser B Biol Sci 275:327–348. https://doi.org/10.1098/rstb.1976.0086
Nagashima T, Oami E, Kutsuna N, Ishiura S, Suo S (2016) Dopamine regulates body size in Caenorhabditis elegans. Dev Biol 412:128–138. https://doi.org/10.1016/j.ydbio.2016.02.021
Fox CA, Mansour A, Watson SJ (1994) The effects of haloperidol on dopamine receptor gene expression. Exp Neurol 130:288–303. https://doi.org/10.1006/exnr.1994.1207
Sora I, Fujiwara Y, Tomita H, Ishizu H, Akiyama K, Otsuki S, Yamamura HI (1992) Lack of effect of haloperidol or methamphetamine treatment on the mRNA levels of two dopamine D, receptor isoforms in rat brain. Psychiatry Clin Neurosci 46:967–973. https://doi.org/10.1111/j.1440-1819.1992.tb02868.x
McCullumsmith RE, Stincic TL, Agrawal SM, Meador-Woodruff JH (2003) Differential effects of antipsychotics on haloperidol-induced vacuous chewing movements and subcortical gene expression in the rat. Eur J Pharmacol 477:101–112. https://doi.org/10.1016/j.ejphar.2003.08.018
Mor DE, Daniels MJ, Ischiropoulos H (2019) The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov Disord 34:167–179
Acknowledgments
We specially acknowledge the collaboration of the colleagues Mahfuzur Miah, Ayodele Jacob Akinyemi, and Tao Ke for technical support.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Aschner M was supported in part by grants from the National Institute of Environmental Health Sciences (NIEHS), R01 ES07331, and R01 ES10563.
Author information
Authors and Affiliations
Contributions
Krum BN: conceptualization, investigation, writing- original draft preparation, conducted all the experiments with the support of Martins AC, Queirós L, Ferrer B, and Milne GL. Soares FAA: conceptualization, writing - review and editing, visualization. Fachinetto R: supervision, writing - review & editing. Aschner M: conceptualization, supervision, resources, writing - review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Krum, B.N., Martins, A.C., Queirós, L. et al. Haloperidol Interactions with the dop-3 Receptor in Caenorhabditis elegans. Mol Neurobiol 58, 304–316 (2021). https://doi.org/10.1007/s12035-020-02124-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02124-9