Abstract
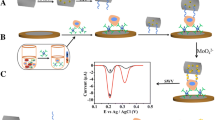
In this study, we fabricated a high-sensitivity “signal-off” electrochemical aptasensing platform for quantifying circulating tumor cells (CTCs) based on target-triggered signal readout of methylene blue (MB). Au nanoparticles (AuNPs) were introduced to enlarge the specific surface area of the gold electrode (GE), which would immobilize homogeneous and more MB-aptamers. MB-modified and stem-loop-like aptamers were assigned as a recognition element with K562 cells. Thiolated complementary strands hybridized with MB-aptamers to form double-stranded DNA (dsDNA) conformation which were further self-assembled on the surface of AuNP-modified GE, leading to a marked current peak of MB signal. In the presence of K562 cells, the MB-aptamers preferred to recognize and bind with the cells, causing the disassembly of MB-aptamers from the GE surface. Therefore, the reduced value of MB signal was related to the number of K562 cells. With the proposed aptasensor, a dynamic linear range from 1 × 102 to 1 × 106 cells mL−1 was obtained with a detection limit of 23 cells mL−1. Moreover, the aptasensor showed good selectivity, stability, and reproducibility as well as potential use in the clinical setting. Meanwhile, characterization techniques such as field-emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, atomic force microscopy, cyclic voltammetry, and electrochemical impedance spectroscopy were performed to analyze the evolution of the morphology and each fabricated step of the constructed aptasensor. Our proposed aptasensor could be designed as a universal platform for CTC determination by replacing tumor cell–specific aptamers, which is a promising strategy for basic research and clinical applications.

Graphical abstract






Similar content being viewed by others
References
Temilola DO, Wium M, Coulidiati TH, Adeola HA, Carbone GM, Catapano CV, et al. The prospect and challenges to the flow of liquid biopsy in Africa. Cells. 2019;8(8).
Rossi G, Ignatiadis M. Promises and pitfalls of using liquid biopsy for precision medicine. Cancer Res. 2019;79(11):2798–804.
Vaidyanathan R, Soon RH, Zhang P, Jiang K, Lim CT. Cancer diagnosis: from tumor to liquid biopsy and beyond. Lab Chip. 2018;19(1):11–34.
Banko P, Lee SY, Nagygyorgy V, Zrinyi M, Chae CH, Cho DH, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol. 2019;12(1):48.
Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16(9):398–406.
Chen L, Bode AM, Dong Z. Circulating tumor cells: moving biological insights into detection. Theranostics. 2017;7(10):2606–19.
Buscail E, Chiche L, Laurent C, Vendrely V, Denost Q, Denis J, et al. Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells. Mol Oncol. 2019;13(9):1811–26.
Kamande JW, Hupert ML, Witek MA, Wang H, Torphy RJ, Dharmasiri U, et al. Modular microsystem for the isolation, enumeration, and phenotyping of circulating tumor cells in patients with pancreatic cancer. Anal Chem. 2013;85(19):9092–100.
Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31(18):1827–40.
Jackson JM, Witek MA, Kamande JW, Soper SA. Materials and microfluidics: enabling the efficient isolation and analysis of circulating tumour cells. Chem Soc Rev. 2017;46(14):4245–80.
Brown HK, Tellez-Gabriel M, Cartron PF, Vallette FM, Heymann MF, Heymann D. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: myth or reality? Drug Discov Today. 2019;24(3):763–72.
Sharma S, Zhuang R, Long M, Pavlovic M, Kang Y, Ilyas A, et al. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol Adv. 2018;36(4):1063–78.
Maduraiveeran G, Sasidharan M, Ganesan V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens Bioelectron. 2018;103:113–29.
Lima HRS, da Silva JS, de Oliveira Farias EA, Teixeira PRS, Eiras C, Nunes LCC. Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosens Bioelectron. 2018;108:27–37.
Yang Y, Fu Y, Su H, Mao L, Chen M. Sensitive detection of MCF-7 human breast cancer cells by using a novel DNA-labeled sandwich electrochemical biosensor. Biosens Bioelectron. 2018;122:175–82.
Ge S, Zhang L, Zhang Y, Liu H, Huang J, Yan M, et al. Electrochemical K-562 cells sensor based on origami paper device for point-of-care testing. Talanta. 2015;145:12–9.
Sun D, Lu J, Chen Z, Yu Y, Mo M. A repeatable assembling and disassembling electrochemical aptamer cytosensor for ultrasensitive and highly selective detection of human liver cancer cells. Anal Chim Acta. 2015;885:166–73.
Chen D, Sun D, Wang Z, Qin W, Chen L, Zhou L, et al. A DNA nanostructured aptasensor for the sensitive electrochemical detection of HepG2 cells based on multibranched hybridization chain reaction amplification strategy. Biosens Bioelectron. 2018;117:416–21.
Wang Y, Chang K, Yang C, Li S, Wang L, Xu H, et al. Highly sensitive electrochemical biosensor for circulating tumor cells detection via dual-aptamer capture and rolling circle amplification strategy. J Biomed Nanotechnol. 2019;15(7):1568–77.
Wang H, Song Y, Chai Y, Yuan R. Highly sensitive biosensor based on target induced dual signal amplification to electrochemiluminescent nanoneedles of Ru(II) complex. Biosens Bioelectron. 2019;140:111344.
Chen Z, Xu Q, Tang G, Liu S, Xu S, Zhang X. A facile electrochemical aptasensor for lysozyme detection based on target-induced turn-off of photosensitization. Biosens Bioelectron. 2019;126:412–7.
Zhu S, Lin X, Ran P, Xia Q, Yang C, Ma J, et al. A novel luminescence-functionalized metal-organic framework nanoflowers electrochemiluminesence sensor via “on-off” system. Biosens Bioelectron. 2017;91:436–40.
Wang H, Zhang B, Zhao F, Zeng B. One-pot synthesis of N-graphene quantum dot-functionalized I-BiOCl Z-scheme cathodic materials for “signal-off” photoelectrochemical sensing of chlorpyrifos. ACS Appl Mater Interfaces. 2018;10(41):35281–8.
Zhou Z, Liu M, Jiang J. The potential of aptamers for cancer research. Anal Biochem. 2018;549:91–5.
Kaur H, Bruno JG, Kumar A, Sharma TK. Aptamers in the therapeutics and diagnostics pipelines. Theranostics. 2018;8(15):4016–32.
Pan Q, Luo F, Liu M, Zhang XL. Oligonucleotide aptamers: promising and powerful diagnostic and therapeutic tools for infectious diseases. The Journal of infection. 2018;77(2):83–98.
Zhou G, Latchoumanin O, Bagdesar M, Hebbard L, Duan W, Liddle C, et al. Aptamer-based therapeutic approaches to target cancer stem cells. Theranostics. 2017;7(16):3948–61.
Cui L, Lu M, Li Y, Tang B, Zhang CY. A reusable ratiometric electrochemical biosensor on the basis of the binding of methylene blue to DNA with alternating AT base sequence for sensitive detection of adenosine. Biosens Bioelectron. 2018;102:87–93.
Xiao Y, Piorek BD, Plaxco KW, Heeger AJ. A reagentless signal-on architecture for electronic, aptamer-based sensors via target-induced strand displacement. J Am Chem Soc. 2005;127(51):17990–1.
Xiao Y, Lubin AA, Heeger AJ, Plaxco KW. Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew Chem. 2005;44(34):5456–9.
Deng C, Pi X, Qian P, Chen X, Wu W, Xiang J. High-performance ratiometric electrochemical method based on the combination of signal probe and inner reference probe in one hairpin-structured DNA. Anal Chem. 2017;89(1):966–73.
Liu LL, Han YW, Zhu KH, Li ZZ, Han YP, Lu Y, et al. Screening and characterization of aptamers of chronic myelognous leukemia K562 cells. Journal of the fourth military medical university. 2009;30(13):1157–60.
Liu LL. Screening, characterization and structure analysis of aptamers binding to chronic myelognous leukemia K562 cells: Lanzhou University (China); 2009.
Meng X, Xu M, Zhu J, Yin H, Ai S. Fabrication of DNA electrochemical biosensor based on gold nanoparticles, locked nucleic acid modified hairpin DNA and enzymatic signal amplification. Electrochim Acta. 2012;71(none):233–8.
Tian M, Pell WG, Conway BE. Nanogravimetry study of the initial stages of anodic surface oxide film growth at Au in aqueous HClO4 and H2SO4 by means of EQCN. Electrochim Acta. 2003;48(18):2675-89.
Zhang X, Xie G, Gou D, Luo P, Yao Y, Chen H. A novel enzyme-free electrochemical biosensor for rapid detection of Pseudomonas aeruginosa based on high catalytic Cu-ZrMOF and conductive Super P. Biosens Bioelectron. 2019;142:111486.
Ma J, Wu L, Li Z, Lu Z, Yin W, Nie A, et al. Versatile electrochemiluminescence assays for PEDV antibody based on rolling circle amplification and Ru-DNA nanotags. Anal Chem. 2018;90(12):7415–21.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81430053, 81401751, 81972027), Chongqing Health Commission (2018QNXM049, 2019ZDXM025), and Medical pre-research project of the Army Medical University (2018XYY04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The experiments were approved by the ethics committee of Southwest Hospital (China).
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1.31 mb)
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, W., Tang, X. et al. Target-triggered “signal-off” electrochemical aptasensor assisted by Au nanoparticle–modified sensing platform for high-sensitivity determination of circulating tumor cells. Anal Bioanal Chem 412, 8107–8115 (2020). https://doi.org/10.1007/s00216-020-02940-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02940-x