Abstract
Purpose
To investigate predictors of treatment interruption and early discontinuation of adjuvant hormonal therapy (HT) in a retrospective cohort of women with newly diagnosed hormone receptor-positive (HR +) breast cancer.
Methods
Eligible cases were identified from a single institutional tumor registry from 2009 to 2015. Patients were followed from initiation of adjuvant HT for a minimum of one year through December 1, 2016. Predictors of treatment interruption or early discontinuation were analyzed with Cox proportional hazards regression models.
Results
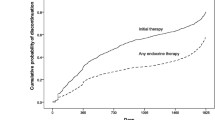
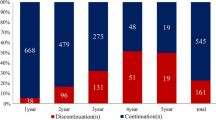
With a median follow-up time of 3.0 years (IQR 1.5–4.5), 22 women (10.9%) discontinued HT early and 47 (23.4%) had at least one treatment interruption of > 14 days. Adjusted Cox proportional hazards regression models showed that women with pre-existing affective disorders were more likely to discontinue therapy early (HR 3.15; 95% CI 1.35–7.37), while those with pre-existing chronic pain disorders were at increased risk for treatment interruption (HR 2.24; 95% CI 1.20–4.19). HT-related symptoms were the most commonly reported reason for HT interruption or discontinuation. Women who experienced severe treatment-related symptoms were at increased risk for both HT interruption (HR 2.64; 95% CI 1.07–6.50) and HT discontinuation (HR 3.48; 95% CI 1.20–10.1).
Conclusions
This study showed that HT interruptions and discontinuation were common, often associated with HT-related symptoms. Clinicians caring for breast cancer patients on HT should monitor closely for treatment-emergent symptoms, especially women with pre-existing disorders, and support them to continue therapy through aggressive symptom management and other patient-centered approaches.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18):1371–1388. https://doi.org/10.1093/jnci/90.18.1371
Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, Smith R, Begovic M, Dimitrov NV, Margolese RG, Kardinal CG, Kavanah MT, Fehrenbacher L, Oishi RH (1999) Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 353(9169):1993–2000. https://doi.org/10.1016/S0140-6736(99)05036-9
Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR (2005) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol 23(3):619–629. https://doi.org/10.1200/JCO.2005.09.121
Carlson RW, Hudis CA, Pritchard KI, National Comprehensive Cancer Network Breast Cancer Clinical Practice Guidelines in Oncology, American Society of Clinical Oncology Technology Assessment on the Use of Aromatase Inhibitors, St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer (2006) Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Cancer Netw 4(10):971–979. https://doi.org/10.6004/jnccn.2006.0082
Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ, American Society of Clinical Oncology (2010) American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28(23):3784–3796. https://doi.org/10.1200/JCO.2009.26.3756
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ (2019) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 37(5):423–438. https://doi.org/10.1200/JCO.18.01160
Early Breast Cancer Trialists' Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0
Early Breast Cancer Trialists' Collaborative Group, Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
Early Breast Cancer Trialists' Collaborative Group (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386(10001):1341–1352. https://doi.org/10.1016/S0140-6736(15)61074-1
Gray R, Early Breast Cancer Trialists' Collaborative Group (2019) Abstract GS3–03: effects of prolonging adjuvant aromatase inhibitor therapy beyond five years on recurrence and cause-specific mortality: an EBCTCG meta-analysis of individual patient data from 12 randomised trials including 24,912 women. Cancer Res 79(4 Supplement):GS3. https://doi.org/10.1158/1538-7445.SABCS18-GS3-03
Barron TI, Cahir C, Sharp L, Bennett K (2013) A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer 109(6):1513–1521. https://doi.org/10.1038/bjc.2013.518
Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126(2):529–537. https://doi.org/10.1007/s10549-010-1132-4
Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C (2013) Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 108(7):1515–1524. https://doi.org/10.1038/bjc.2013.116
Chlebowski RT, Geller ML (2006) Adherence to endocrine therapy for breast cancer. Oncology 71(1–2):1–9. https://doi.org/10.1159/000100444
Dunn J, Gotay C (2013) Adherence rates and correlates in long-term hormonal therapy. Vitam Horm 93:353–375. https://doi.org/10.1016/B978-0-12-416673-8.00003-4
Banning M (2012) Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care (Engl) 21(1):10–19. https://doi.org/10.1111/j.1365-2354.2011.01295.x
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134(2):459–478. https://doi.org/10.1007/s10549-012-2114-5
Land SR, Walcott FL, Liu Q, Wickerham DL, Costantino JP, Ganz PA (2016) Symptoms and QOL as predictors of chemoprevention adherence in NRG oncology/NSABP Trial P-1. J Natl Cancer Inst 108(4):365. https://doi.org/10.1093/jnci/djv365
Smith SG, Sestak I, Howell A, Forbes J, Cuzick J (2017) Participant-reported symptoms and their effect on long-term adherence in the international breast cancer intervention Study I (IBIS I). J Clin Oncol 35(23):2666–2673. https://doi.org/10.1200/JCO.2016.71.7439
Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA (2004) Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor–positive breast cancer. J Clin Oncol 22(16):3309–3315. https://doi.org/10.1200/JCO.2004.11.064
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99(2):215–220. https://doi.org/10.1007/s10549-006-9193-0
Atkins L, Fallowfield L (2006) Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer 42(14):2271–2276. https://doi.org/10.1016/j.ejca.2006.03.004
Grunfeld EA, Hunter MS, Sikka P, Mittal S (2005) Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns 59(1):97–102. https://doi.org/10.1016/j.pec.2004.10.005
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, Stearns V, Hayes DF, Storniolo AM (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30(9):936–942. https://doi.org/10.1200/JCO.2011.38.0261
Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N (2006) Side effects of aromatase inhibitors versus tamoxifen: the patients' perspective. Am J Surg 192(4):496–498. https://doi.org/10.1016/j.amjsurg.2006.06.018
Green F, Page D, Fleming I, Fritz A, Balch C, Haller D, Morrow M (2002) American Joint Commission on Cancer (AJCC) cancer staging manual, 6th edn. Springer, pp 223–240. https://cancerstaging.org/referencestools/deskreferences/Documents/AJCC6thEdCancerStagingManualPart1.pdf. Accessed 22 May 2020
Dong J, Esham KS, Boehm L, Karim SA, Lin M, Mao D, Wang F, Fein D, Wang H, Studenmund C (2020) Timeliness of treatment initiation in newly diagnosed breast cancer patients. Clin Breast Cancer 20(1):e27–e35. https://doi.org/10.1016/j.clbc.2019.06.009
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619. https://doi.org/10.1016/0895-4356(92)90133-8
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium RE (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208. https://doi.org/10.1016/j.jbi.2019.103208
De Vos FY, van Laarhoven HW, Laven JS, Themmen AP, Beex LV, Sweep CG, Seynaeve C, Jager A (2012) Menopausal status and adjuvant hormonal therapy for breast cancer patients: a practical guideline. Crit Rev Oncol Hematol 84(2):252–260. https://doi.org/10.1016/j.critrevonc.2012.06.005
Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ (2012) Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 97(4):1159–1168. https://doi.org/10.1210/jc.2011-3362
Hoagland AC, Morrow GR, Bennett JM, Carnrike CL Jr (1983) Oncologists' views of cancer patient noncompliance. Am J Clin Oncol 6(2):239–244. https://doi.org/10.1097/00000421-198304000-00018
Demissie S, Silliman RA, Lash TL (2001) Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol 19(2):322–328. https://doi.org/10.1200/JCO.2001.19.2.322
Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ (2007) Early discontinuation of tamoxifen: a lesson for oncologists. Cancer 109(5):832–839. https://doi.org/10.1002/cncr.22485
Bender CM, Gentry AL, Brufsky AM, Casillo FE, Cohen SM, Dailey MM, Donovan HS, Dunbar-Jacob J, Jankowitz RC, Rosenzweig MQ, Sherwood PR, Sereika SM (2014) Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum 41(3):274–285. https://doi.org/10.1188/14.ONF.274-285
Stanton AL, Petrie KJ, Partridge AH (2014) Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat 145(2):525–534. https://doi.org/10.1007/s10549-014-2961-3
Brito C, Portela MC, de Vasconcellos MT (2014) Adherence to hormone therapy among women with breast cancer. BMC Cancer 14:397. https://doi.org/10.1186/1471-2407-14-397
Aiello Bowles EJ, Boudreau DM, Chubak J, Yu O, Fujii M, Chestnut J, Buist DS (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract 8(6):e149–157. https://doi.org/10.1200/JOP.2012.000543
Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM (2007) Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care 45(5):431–439. https://doi.org/10.1097/01.mlr.0000257193.10760.7f
Owusu C, Buist DS, Field TS, Lash TL, Thwin SS, Geiger AM, Quinn VP, Frost F, Prout M, Yood MU, Wei F, Silliman RA (2008) Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol 26(4):549–555. https://doi.org/10.1200/JCO.2006.10.1022
He W, Fang F, Varnum C, Eriksson M, Hall P, Czene K (2015) Predictors of discontinuation of adjuvant hormone therapy in patients with breast cancer. J Clin Oncol 33(20):2262–2269. https://doi.org/10.1200/JCO.2014.59.3673
Cella D, Fallowfield LJ (2008) Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 107(2):167–180. https://doi.org/10.1007/s10549-007-9548-1
Dent SF, Gaspo R, Kissner M, Pritchard KI (2011) Aromatase inhibitor therapy: toxicities and management strategies in the treatment of postmenopausal women with hormone-sensitive early breast cancer. Breast Cancer Res Treat 126(2):295–310. https://doi.org/10.1007/s10549-011-1351-3
Hadji P (2010) Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol 73(2):156–166. https://doi.org/10.1016/j.critrevonc.2009.02.001
Monnier A (2007) Clinical management of adverse events in adjuvant therapy for hormone-responsive early breast cancer. Ann Oncol 18(Suppl 8):viii36–viii44. https://doi.org/10.1093/annonc/mdm264
Mortimer JE (2010) Managing the toxicities of the aromatase inhibitors. Curr Opin Obstet Gynecol 22(1):56–60. https://doi.org/10.1097/GCO.0b013e328334e44e
Mouridsen HT (2006) Incidence and management of side effects associated with aromatase inhibitors in the adjuvant treatment of breast cancer in postmenopausal women. Curr Med Res Opin 22(8):1609–1621. https://doi.org/10.1185/030079906X115667
Massacesi C, Zepponi L, Rocchi M, Rossini S, Burattini L (2006) Tamoxifen-related endocrine symptoms in early breast cancer patients are relieved when it is switched to anastrozole. J Clin Oncol 24(18_suppl):10597–10597. https://doi.org/10.1200/jco.2006.24.18_suppl.10597
Guth U, Myrick ME, Schotzau A, Kilic N, Schmid SM (2011) Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: how often and how often does it work? Breast Cancer Res Treat 129(3):799–807. https://doi.org/10.1007/s10549-011-1668-y
Acknowledgements
The project described was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number TL1TR001062. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding
Additional support was provided by the Susan G. Komen for the Cure Training Grant in Health Disparities (GTDR15333918) and the American Cancer Society (CRP-17–112-06-COUN).
Author information
Authors and Affiliations
Contributions
DM and HH participated in study design, data collection, and analysis. They also participated in the interpretation of the results and development of the manuscript. HC oversaw the analysis plan and critically reviewed the manuscript. DD, JD, and MW participated in data collection and critically reviewed the manuscript. JKE and KMF critically reviewed the manuscript. HC, JKE, and KMF were apprised from the outset on study design, patient selection, and data collection. SKP supervised all aspects of the study, including design, data collection, analysis, interpretation, and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Research involving human and animal rights
The research did involve human subjects, based on the study design and retrospective review of the electronic health records.
Informed consent
The Tufts Medical Center Institutional Review Board granted a waiver of consent and a HIPAA waiver of research authorization for the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mao, D., Hachem, H., Chang, H. et al. Treatment interruption and discontinuation of hormonal therapy in hormone receptor-positive breast cancer patients. Breast Cancer Res Treat 184, 665–674 (2020). https://doi.org/10.1007/s10549-020-05892-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05892-z