- 1Engineering Research Center of Molecular and Neuro Imaging of the Ministry of Education, School of Life Sciences and Technology, Xidian University, Xi’an, China
- 2School of Electronics and Information, Xi’an Polytechnic University, Xi’an, China
- 3Department of Radiology, Xijing Hospital, The Fourth Military Medical University, Xi’an, China
It has been reported that one night of acute sleep deprivation (SD) could induce brain structural changes at the synaptic and neuronal levels in animal studies, and could lead to white matter microstructure and cortical thickness change in human neuroimaging studies. In this study, we focused on changes of brain gray matter density (GMD) after one night of acute SD, which has not been explored previously. Twenty-three normal young participants completed the experiment. Each participant underwent twice T1-weighted structural image scanning with one at 08:00 after normal sleep [resting wakeful (RW)] and the other at 08:00 after 24 h of SD. Using voxel-based morphometry (VBM) analysis by FSL-VBM software, we compared GMD between RW and SD. In addition, the gray matter volume (GMV) and cortical thickness (CT) were also calculated based on volumetric and surface measures with FreeSurfer software. The psychomotor vigilance test (PVT) and the Karolinska Sleepiness Scale (KSS) were performed and evaluated for correlation analysis with GMD, GMV, and CT of the significant regions. Our results showed that the GMD in the right frontal pole (FP), right superior frontal gyrus (SFG), and right middle frontal gyrus significantly increased and GMV and CT in the right temporal pole (TP) significantly decreased after 24 h of acute SD. SD-induced changes in GMD in the right middle frontal gyrus were positively correlated with the changes of KSS scores (Spearman’s correlation r = 0.625, p = 0.0014, Bonferroni correction with p < 0.05/25). Taken together, our findings suggested that one night of acute SD could induce substantial brain structure changes and the alterations in GMD in the right middle frontal gyrus (MFG) might be implicated in sleepiness after SD.
Introduction
Sleep deprivation (SD) has been found to cause functional abnormalities in the human brain (Krause et al., 2017). One night or 24 h of SD can result in significant changes in brain activation which is necessary to perform cognitive tasks, such as working memory, attention, and inhibition control (Chee and Chuah, 2007; Muto et al., 2012; Zhao et al., 2019a). Furthermore, resting-state brain functional activities, such as functional metabolism and functional connectivity, are significantly impacted by acute SD (Akerstedt and Gillberg, 1990; Killgore et al., 2015; Yeo et al., 2015; Xu et al., 2016; Motomura et al., 2017; Zhao et al., 2019b). However, the effect of one night of acute SD on human brain structure has not been thoroughly characterized.
Short-term changes in brain structure after SD have been first observed in animal sleep brain plasticity studies. Previous studies have indicated that sleep is important for cell membranes and myelin maintenance in the brain (Cirelli et al., 2004; Mongrain et al., 2010), and that these structures are susceptible to be damaged by sleep loss (Hinard et al., 2012; Bellesi et al., 2013). Furthermore, recent ultrastructural studies have reported the change of synapse structural plasticity under normal wake and sleep state alternations (Cirelli, 2013; Frank and Cantera, 2014; Areal et al., 2017), the increase of synaptic puncta and spine numbers in fruit flies (Bushey et al., 2011; Liu et al., 2016), and decrease of spine density and dendrite length in rodents after SD (Acosta-Pena et al., 2015; Havekes et al., 2016; Areal et al., 2017). These findings suggest that acute SD may affect brain structure at synaptic and neuronal levels. Besides animal studies, two recent neuroimaging studies have reported significant changes in human white matter and cortical thickness (CT) after one night of acute SD. Using diffusion tensor MRI (DTI), Elvsashagen et al. (2015) found that 23 h of SD caused a remarkable reduction in axial diffusivity, radial diffusivity, and mean diffusivity compared with rested wakefulness (RW) state. Acute SD has also been found to result in the decrease of CT in the bilateral medial parietal cortex (Elvsashagen et al., 2017). Taken together, these animal and human studies indicate that brain structure is susceptible to be changed by acute SD. In addition to white matter integrity and CT, gray matter density (GMD), a structural T1-weighted morphometry measure, is another commonly used index for structural plasticity in neuroimaging measurements (Thomas and Baker, 2013). So far, the impact of SD on GMD is still unclear. Investigation about how GMD is altered after SD would improve our understanding about the brain resistance to sleep loss.
Therefore, in our present study, we explored the effect of 24 h of SD on brain GMD in 23 normal young participants using voxel-based morphometry (VBM). The relationship between GMD change (△GMD) and alterations in vigilant attention [as measured by the psychomotor vigilance test (PVT)] and sleepiness [as measured by the Karolinska Sleepiness Scale (KSS)] after SD were also evaluated. In addition, the gray matter volume (GMV) and CT were also calculated.
Materials and Methods
Subjects
The recruitment criteria and experimental procedure were similar to those in our previous researches (Zhu et al., 2017; Zhao et al., 2018). Twenty-five subjects were recruited according to the following inclusion criteria: (1) right handed and healthy; (2) regular sleep schedules of 7–9 h per night, between 22:00 and 08:00; (3) no history of alcohol or drugs abuse; (4) be free of any self-reported medical, psychiatric, neurological, or sleep disorders; (5) not presenting an extreme morning or extreme evening type, assessed by the Munich Chronotype Questionnaire (Chen et al., 2016). One subject was excluded because of the MRI scanner failure. The other subject opted out of this study during the SD session. The final analyzed group consisted of 23 subjects [mean age 20.30 ± 1.64 years; range 17–23; 10 males and 13 females; body mass index (BMI) 21.45 ± 3.25].
All subjects declared that they did not smoke or consume any stimulants, medications, alcohol, or caffeine for at least 24 h before the MRI scanning and provided written informed consent before participation. All research procedures were conducted in accordance with the Declaration of Helsinki and approved by the institutional research ethics committee of the Xijing Hospital of the Air Force Medical University.
Experimental Procedure
All participants visited the laboratory three times. During the first visit, participants underwent a screening process. They were briefed about the experimental procedure and given instructions about the PVT. Participants were scheduled for the second visit 1 week later. During the second visit, subjects underwent the MR scanning at 08:00 under one of the two states (RW and 24 h of SD) after performing PVT task and rating the sleepiness by KSS. During the third visit, subjects underwent the MR scanning at 08:00 under the other state after KSS and PVT performance. To minimize possible residual effects of SD on cognition (Van Dongen et al., 2003), the state of RW or 24 h of SD was scheduled in a randomized, cross-over fashion with at least 1 week apart. For the SD state, participants were monitored by experimenters and were not allowed to fall asleep from 22:00 to 08:00. They were allowed to engage in non-strenuous activities such as reading and watching videos. For the RW state, subjects were arranged to perform regular sleep. Furthermore, participants were asked to continue their usual daily activities, but were not allowed to engage in shiftwork or stay up all night during the interval between the second and third visits.
Behavioral Acquisition
We used PVT to measure vigilant attention, which is the cognitive domain most severely impaired by SD (Lim and Dinges, 2008; Yang et al., 2018). This task used in the present study was adapted from our previous research (Zhu et al., 2017, 2018). First, a red fixation cross appeared in the center of a black background on the screen and remained for 2 s. Then, the red fixation cross disappeared, and the black background screen was presented for a random duration of 2–10 s. After that, a red target circle was displayed and participants were instructed to press a button as quickly as possible with their right index finger. They were required to press the button within 30 s. If the participant responded, the red target circle disappeared and the real-time reaction time (RT) was displayed on the screen to provide feedback regarding their performance. The feedback was presented 1 s after the response. If the participant did not respond, the displayed real-time RT was 0 ms. This task lasted for 7 min.
The primary behavioral measurements of interest in the PVT were (1) lapse ratio (the lapse was defined as the trail with RT > 500 ms, and lapse ratio was defined as the number of lapse/trails number); (2) the median RT of all trials; (3) the reciprocal of RT of the fastest 10% trials, labeled “10% fast 1/RT”; and (4) the reciprocal of RT of the slowest 10% trials, labeled “10% slow 1/RT.”
We also assessed sleepiness, the main consequence of insufficient sleep (Akerstedt et al., 2014), using the KSS under RW or SD state. The KSS is a nine-point scale and participants rated sleepiness by circling a number from 1 (very alert) to 9 (very sleepy, fighting sleep) that represented their experience of sleepiness (Akerstedt and Gillberg, 1990).
MRI Data Acquisition
MRI scanning was performed in a 3T GE MR750 scanner at Department of Radiology, Xijing Hospital, The Air Force Medical University, Xi’an, China. The 3D T1-weighted structural data were obtained using the following parameters: repetition time = 8.2 ms; matrix = 512 × 512; echo time = 3.18 ms; in-plane resolution = 0.5 × 0.5 mm2; field of view = 256 × 256 mm2; sagittal slices = 196; slice thickness = 1 mm; flip angle = 9°.
Voxel-Based Morphometry Analysis
The FSL version 5.0.41 was used for VBM analysis (Jenkinson et al., 2012). First, all subjects’ T1-weighted data were brain extracted using the Brain Extraction Tool (BET) (Smith, 2002) and visually checked by an experienced neurologist to remove any leftover non-brain tissue. Second, brain-extracted data were segmented into gray matter, white matter, and cerebrospinal fluid using the FMRIB’s Automated Segmentation Tool (FAST) (Zhang et al., 2001). Third, the obtained gray matter data were non-linearly registered to the gray matter ICBM-152 template using the FMRIB’s Non-linear Image Registration Tool (FNIRT). Fourth, a symmetric, study-specific gray matter template was generated by averaging the registered images. Fifth, the segmented gray matter data of each subject were non-linearly registered to the aforementioned obtained gray matter template and modulated using the Jacobian of the warp field to produce maps of GMD. Finally, the resulting GMD images were smoothed with a Gaussian kernel of 3 mm (a full-width half-maximum of ∼7 mm).
Cortical Thickness and Volume Analysis
The FreeSurfer longitudinal pipeline version 5.3 was used for brain volume segmentation and cortical reconstruction2. Using robust, inverse consistent registration (Reuter et al., 2010), an unbiased within-subject template space and image was created (Reuter and Fischl, 2011; Reuter et al., 2012). Several processing steps such as skull stripping, Talairach transforms, atlas registration, as well as spherical surface maps and parcellations were then initialized with common information from the within-subject template, significantly increasing reliability and statistical power. Then, an additional manual checking step was applied to improve the skull stripping of within-subject templates (see surfer.nmr.mgh.harvard.edu/fswiki/ LongitudinalProcessing for details).
Based on the Desikan/Killiany atlas, the mean value of each volume or thickness segmentation was calculated. For GMV segmentations, 74 left and 74 right cortical gray matter regions, as well as seven left and seven right subcortical gray matter regions were analyzed (for more details, see surfer.nmr.mgh.harvard.edu/fswiki/MorphometryStats). For CT segmentations, 74 left and 74 right cortical gray matter regions were analyzed (for details, see surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation).
Statistical Analysis
In order to explore the effect of SD on the GMD, we compared the GMD maps between RW and SD states using a permutation-based non-parametric paired t-test statistical analysis with Threshold-Free Cluster Enhancement (TFCE) correction for multiple comparisons (p < 0.05). The significant brain regions were identified according to the Harvard–Oxford cortical and subcortical structural atlas (Desikan et al., 2006). The effect of SD on the GMV and CT was evaluated in 162 and 148 brain regions, respectively. Bonferroni correction was used for multiple comparisons (p = 0.05/310). Then, we used the significant brain regions as regions of interest (ROIs) and calculated the GMD, GMV, or CT changes (△GMD, △GMV, or △CT) of these ROIs between these two states (SD-RW). Pearson correlation analysis was performed between △GMD, △GMV, or △CT and the changes (SD-RW) of lapse ratio, median RT, 10% fast 1/RT, and 10% slow 1/RT of PVT. Spearman’s correlation analysis was performed between the △GMD and the △KSS. Age, gender, and BMI were controlled for each correlation analysis. The significant correlation was identified with the Bonferroni correction [p < 0.05/(5∗n); n is the number of ROIs and 5 is the number of behavioral measurements].
Results
Behavioral Results
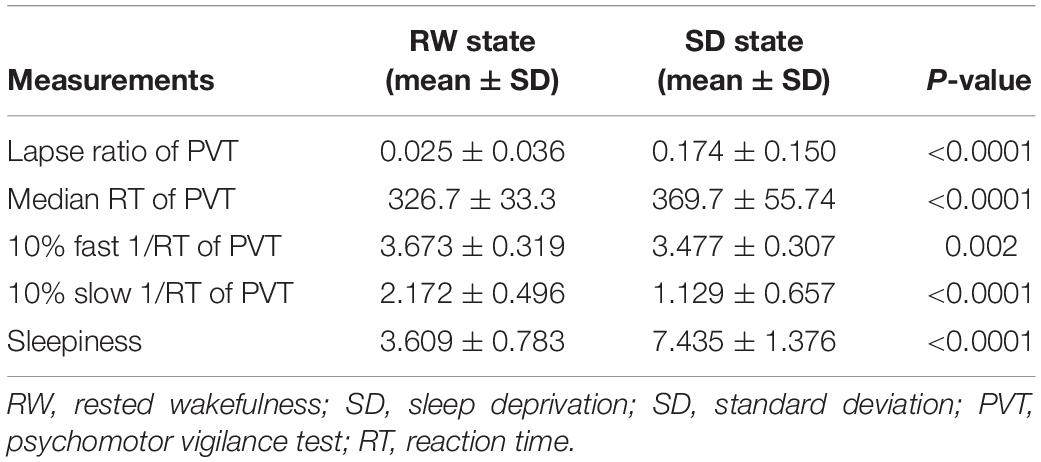
After SD, participants exhibited a significant increase in KSS score (Table 1). Poorer performance on the PVT was also observed after SD with increased lapse ratio (p < 0.0001), slower median RT (p < 0.0001), decreased 10% fast 1/RT (p < 0.002), and decreased 10% slow 1/RT (p < 0.0001) (Table 1 and Figure 1). The change of each measurement of PVT was not correlated with the change of sleepiness (p-values = 0.782, 0.332, 0.387, and 0.654 for lapse ratio, median RT, 10% fast 1/RT, and 10% slow 1/RT of PVT, respectively).
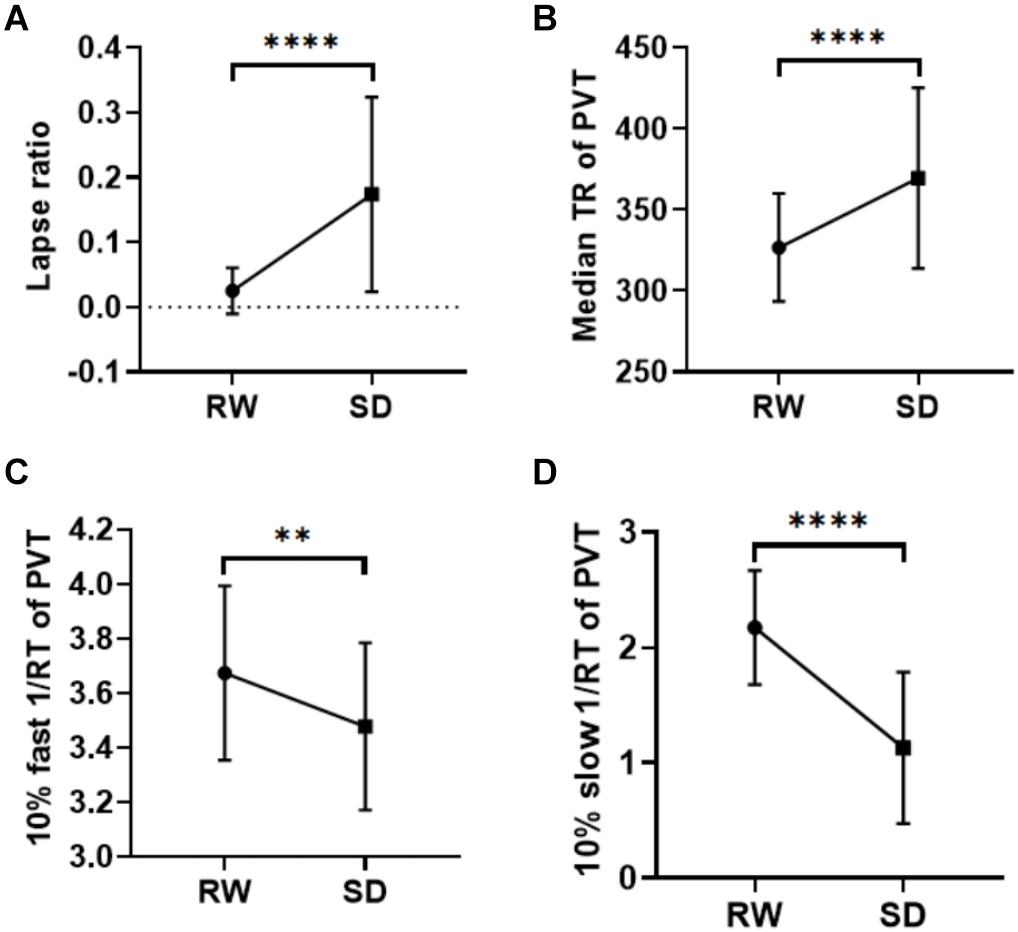
Figure 1. Behavioral results. After SD, participants showed more lapse (p < 0.0001, A), a slower median RT in the PVT (p < 0.0001, B), a decreased 10% fast 1/RT (p < 0.002, C), and reduced 10% slow 1/RT (p < 0.0001, D). **p < 0.01, ****p < 0.0001. RW, rested wakefulness; SD, sleep deprivation; KSS, Karolinska Sleepiness Scale; PVT, psychomotor vigilance test; RT, reaction time.
GMD Changes After SD
Significant increase of GMD was observed in several regions (Figure 2) after SD with TFCE correction (p < 0.05), including the right frontal pole (FP), the right superior frontal gyrus (SFG), and the right middle frontal gyrus (MFG). There were no regions that exhibited reduced GMD after SD.
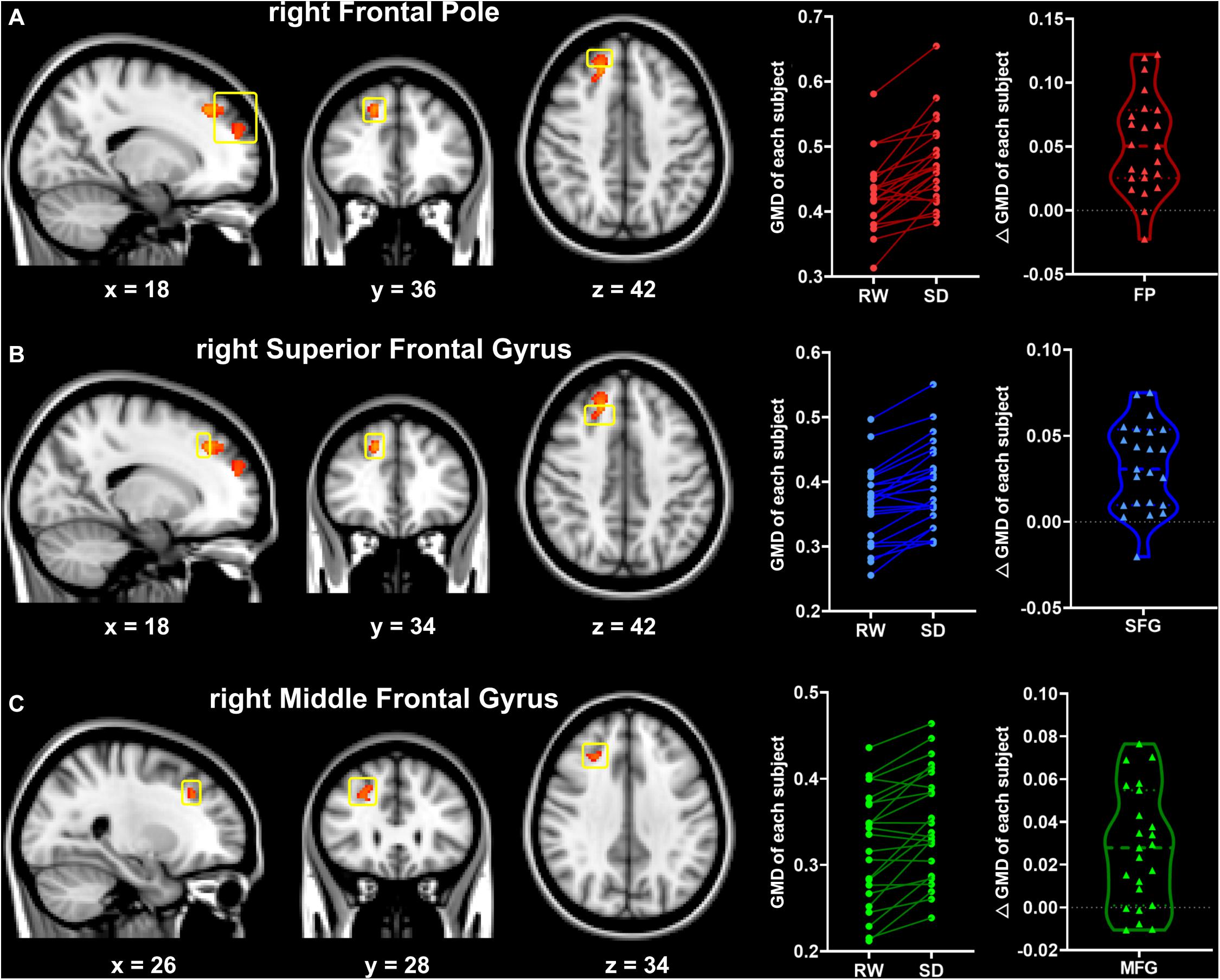
Figure 2. Significant changes in gray matter density between the RW and SD states (RW < SD, TFCE-corrected, p < 0.05). Significant brain regions included the right frontal pole (FP, A), the right superior frontal gyrus (SFG, B), and the right middle frontal gyrus (MFG, C). The yellow box indicates the significant brain regions. The numbers at the bottom indicate the MNI coordinates of the slices. RW, rested wakefulness; SD, sleep deprivation.
GMV and CT Changes After SD
Gray matter volume in the right temporal pole (TP) significantly decreased after SD (RW: 7003 ± 728 vs. SD: 6235 ± 806, t = 6.714, p < 0.0001, Bonferroni correction with p < 0.05/310 = 0.00161, Figures 3A,B), and it did not show significant changes in other brain regions. Similarly, CT measurement significantly decreased only in the right TP after SD (RW: 3.37 ± 0.18 vs. SD: 3.19 ± 0.22, t = 4.988, p < 0.0001, Bonferroni correction with p < 0.05/310 = 0.000161, Figures 3C,D).
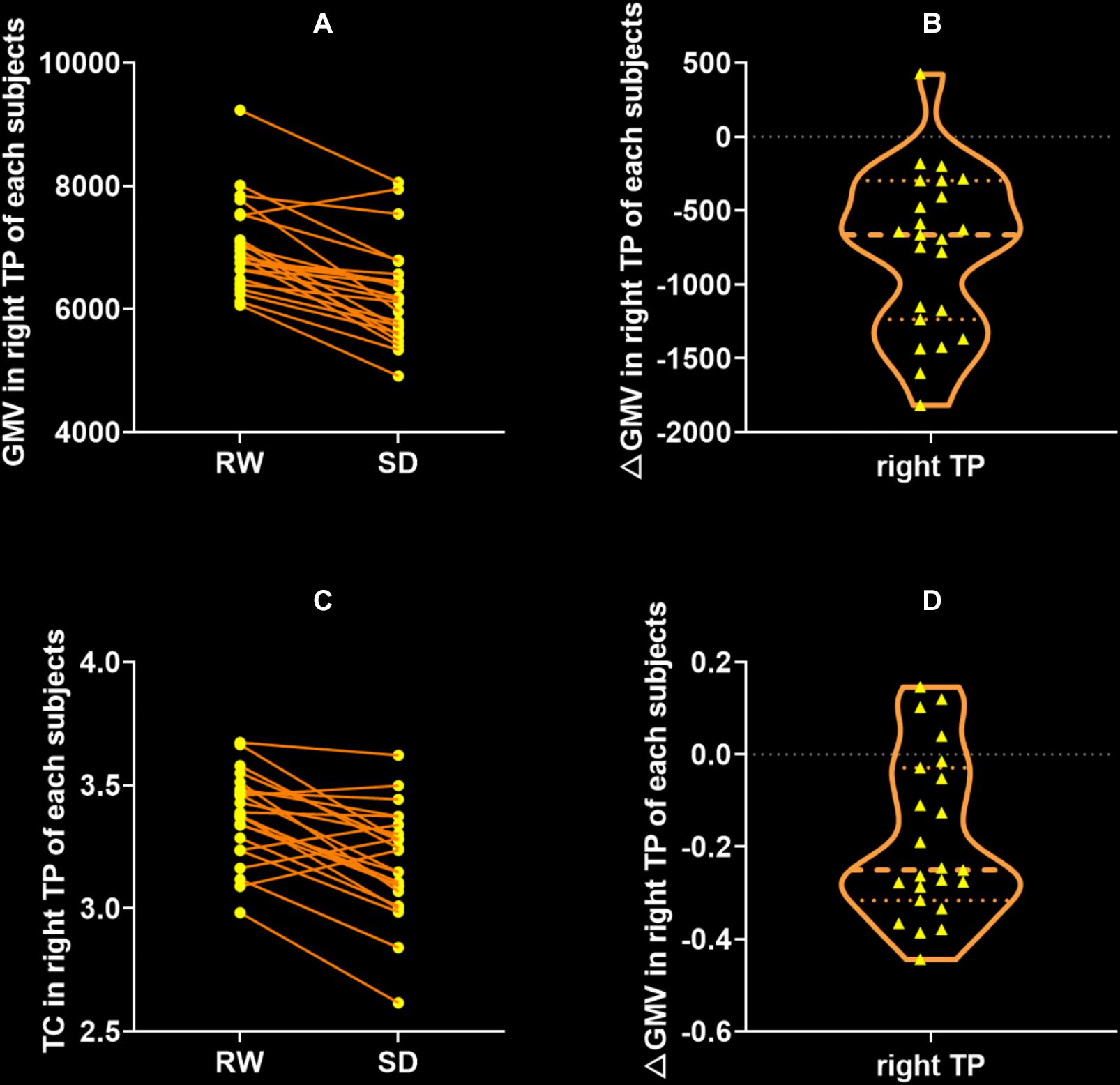
Figure 3. Significant decrease of gray matter volume (GMV) and cortical thickness (CT) between after SD state. The only significant brain region of GMV (A,B) and CT (C,D) was the right temporal pole (TP). There were no regions that exhibited significant change of GMV or CT after SD. RW, rested wakefulness; SD, sleep deprivation.
Relationship Between Brain Structure Changes and Behavioral Performance
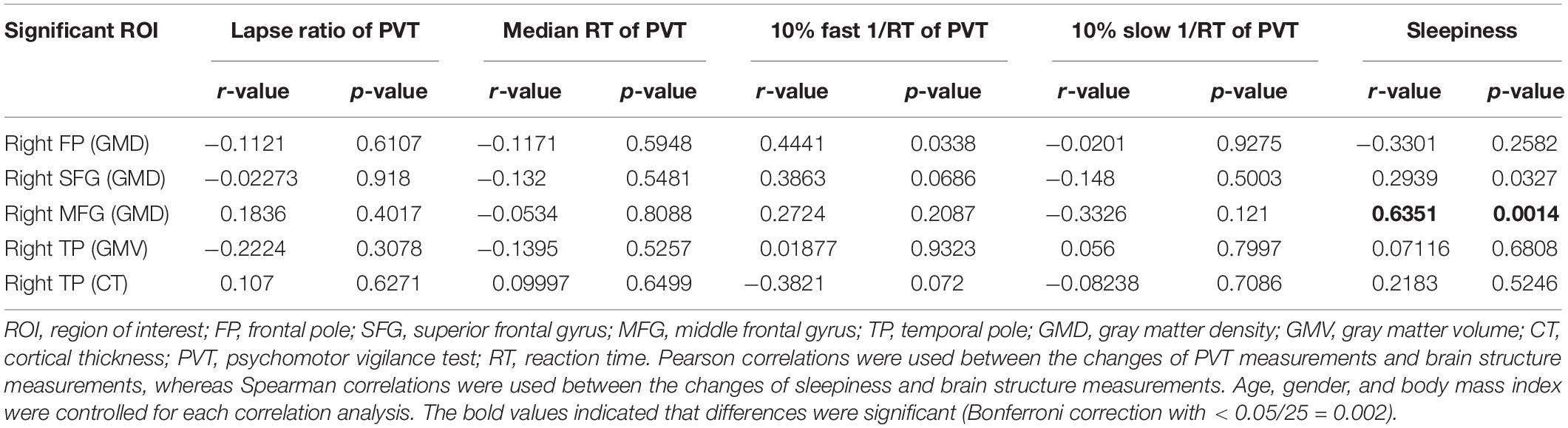
To examine the relationship between the differences between gray structure of the brain and behavioral performance, we correlated △GMD, △GMV, or △CT in the aforementioned significant brain regions with the performance changes of PVT or KSS. Factors of age, gender, and BMI were controlled. All of the correlation results are presented in Table 2. According to the results, △GMD in the MFG and △KSS scores showed significantly positive correlation, as shown in Figure 4 (Spearman’s correlation r = 0.625, p = 0.0014, Bonferroni correction with p < 0.05/25).
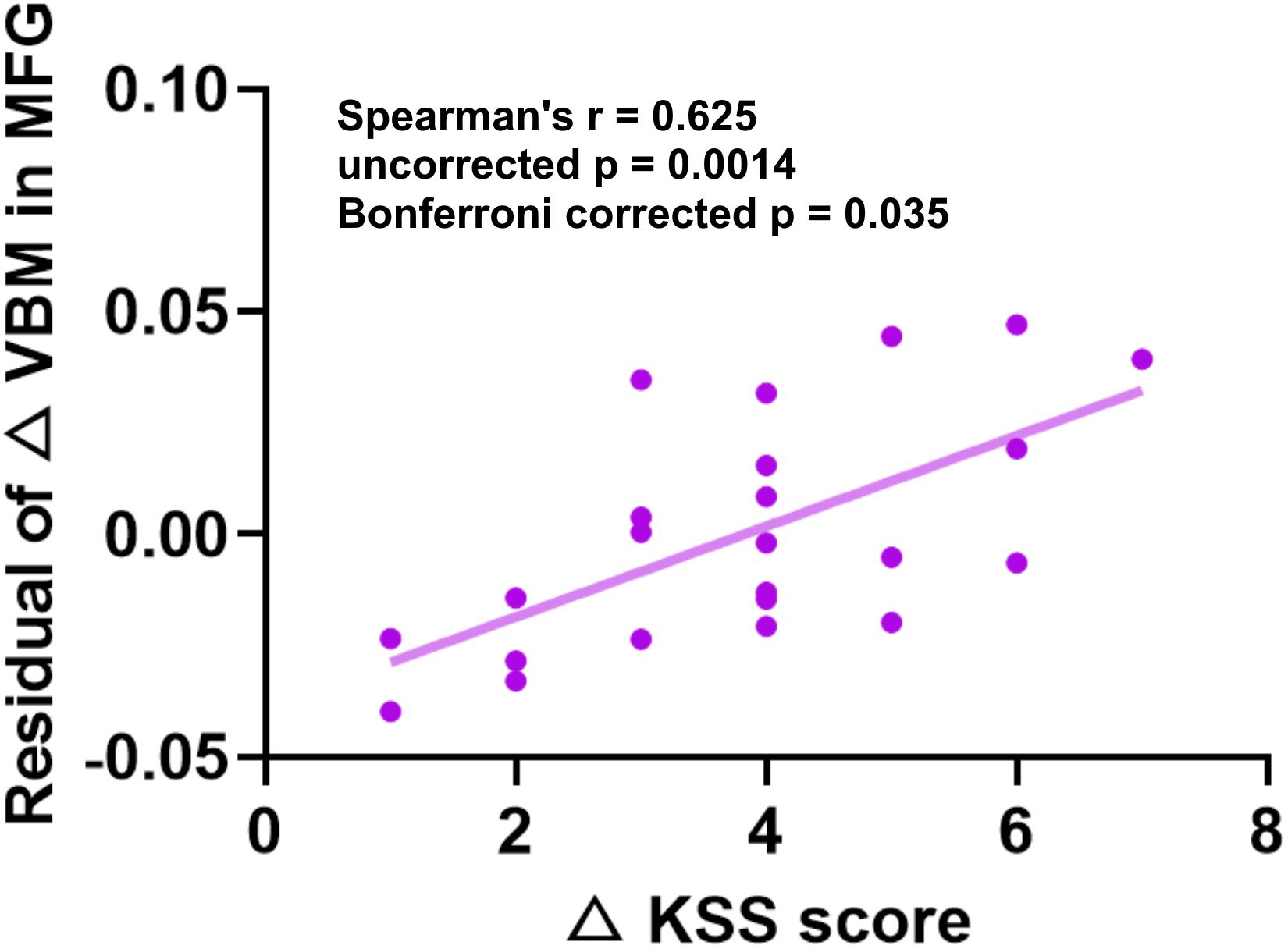
Figure 4. Significant positive Spearman’s correlations between GMD changes in MFG and KSS changes. KSS, Karolinska Sleepiness Scale; GMD, gray matter density; MFG, middle frontal gyrus.
Discussion
In the present study, we investigated the effect of 24 h of SD on GMD using the VBM. We found that GMD in the right FP, the right SFG, and the right MFG significantly increased and that GMV and CT in the right TP significantly decreased after SD. Besides, SD could significantly increase the level of sleepiness and decrease the level of vigilant attention. After controlling factors of gender, age, and BMI, △GMD in the MFG and △KSS scores showed significantly positive correlation. These results suggested that SD could induce alterations in brain structure, which might contribute to the behavioral performance changes.
To our knowledge, this is the first evidence of voxel-wise GMD changes after 24 h of acute SD. Increases of GMD in the right FP, right SFG, and right MFG observed in the study were consistent with Horne’s hypothesis that the PFC was vulnerable to SD (Horne, 1993). The PFC is engaged in attention, working memory, and most of the complex cognitive processes (Whitney et al., 2019). A meta-analytic review of the neural mechanisms underlying vigilant attention identified that the right-lateralized mid- and ventrolateral PFC was one of the core networks engaged during vigilant attention in humans (Langner and Eickhoff, 2013).
Increase of GMD after SD might be related with the Synaptic Homeostasis Hypothesis (Tononi and Cirelli, 2014). As we know, sleep plays a crucial role in the processes associated with learning, memory, and brain plasticity (Diekelmann and Born, 2010; Abel et al., 2013; Rasch and Born, 2013). Recent animal studies have documented the growth of synaptic strength during the waking state, even over a period of a few hours (Bushey et al., 2011). For humans, learning and memory associated with mental and physical activities are common daytime processes and could induce a net increase in synaptic strength in many neuronal circuits while synaptic strength is downscaled to baseline level during sleep (Bushey et al., 2011; Tononi and Cirelli, 2014; Diering, 2017). This downsizing helps sustain energy, make efficient use of gray matter space, and enable synapses to be reused for future memory encoding (Tononi and Cirelli, 2006). However, if participants underwent SD, this downsizing of synaptic strength would be hindered, leading to an increase of synaptic strength oppositely. These changes may further lead to inflation of GMD in the brain macrostructure. Therefore, SD may induce the increase of GMD because of blocking the synapse pruning process.
Sleepiness is the main consequence of sleep loss. Positive correlations between the ΔGMD in the MFG and the changes of sleepiness indicated that participants with greater increase of GMD in the MFG tended to have higher levels of sleepiness after SD. Previous study found that increase in the beta-frequency band power in the MFG was positively associated with sleepiness (Tanaka et al., 2014) and the fatigue score significantly correlated with gray matter atrophy in the MFG in multiple sclerosis (Sepulcre et al., 2009; Fuchs et al., 2019). Thus, the accumulation of daytime activities might be leading to the increase in the beta-frequency band power and synaptic strength in the MFG. Considering the previous evidence that SD might prevent the downscaling of synaptic strength growth to baseline in the PFC and the result of sleepiness increase after SD observed in our study, the correlations between the △GMD in the MFG and the changes of sleepiness in this study might be a reflection of this neural mechanism. What is more, Elvsashagen et al. (2015) have investigated the effect of one night of SD on cerebral white matter DTI parameters and found that widespread axial diffusivity decreased after SD and that larger decreased axial diffusivity was correlated with greater levels of sleepiness after SD. They also found significant cortical thinning in the bilateral medial parietal cortex after SD, and noted that greater thinning was associated with a higher level of sleepiness after SD (Elvsashagen et al., 2017). These results suggest that, besides GMD, sleepiness could also be associated with CT and DTI indices. Further research could explore whether these changes in brain structure represent the neural basis of increased sleepiness after SD.
Besides GMD increase, significant decreases of GMV and CT in the right TP after SD were also observed (Figure 3). As previously reported, the structural and functional changes in TP were associated with the poor sleep quality in normative aging (Amorim et al., 2018) and insomnia (Santarnecchi et al., 2018). The plasticity in the right TP is also involved in the sleep-dependent motor memory (Walker et al., 2005a) and visual skill learning (Walker et al., 2005b). Considering that CT in the bilateral medial parietal cortex significantly decreased after SD (Elvsashagen et al., 2017), the significant decrease in GMV and CT in the TP in the study may reflect the inhibition of sleep-dependent activities induced by acute SD.
The brain structure changes found in this study were induced by acute total sleep loss. Bernardi et al. (2016) have investigated the effect of the intensive, prolonged task training on structural indices. They found decreased cortical mean diffusivity, declined ventricular volume, and increased gray and white matter subcortical volumes after 24 h of task practice combined with SD, but all changes reverted after 8 h of sleep recovery. Therefore, the alterations of GMD, GMV, and CT after acute SD may be neurobiologically temporary effects and could be reverted following 1 day or few days of recovery sleep. However, numerous studies have documented that sleep disorders and psychiatric disorders comorbid with sleep loss are associated with the brain gray matter abnormalities. Previous structural MRI studies have demonstrated the gray matter atrophy/inflation in patients with chronic insomnia (Dang-Vu, 2013; Tahmasian et al., 2018). How acute SD-induced reversible brain structural changes develop into long-term sleep problem-induced irreversible brain structural damage is an important topic to be studied and explored in the future.
Several limitations should be considered when interpreting the present findings. First, it is known that hydration affects brain structure easily (Dickson et al., 2005; Duning et al., 2005; Kempton et al., 2009; Streitburger et al., 2012), which is hard to be avoided. In our experimental design, we asked participants not to deliberately avoid water intake or to drink too much water during SD period and in the morning after normal sleep. Although the impact of hydration on the results could not be completely ruled out, we have minimized the impact of hydration. Besides, because a previous study argued that ventricular volume is more sensitive to dehydration or hyperhydration than GMV (Streitburger et al., 2012), we analyzed the changes in ventricular volume and no significant difference in ventricular volume was observed between the SD and RW periods (p = 0.178). Taken together, we believed that the changes in GMD in this study were unlikely caused by hydration. Second, we only compared the GMD after normal sleep with that after 24 h of SD and found significant brain structure changes after SD. However, the recovery effect on these short-term brain structure changes is unclear. Bernardi et al. (2016) reported that brain structure could be restored after recovery sleep. Further studies are required to explore the restoration effect of sleep recovery on brain structure. Third, recent studies have reported that behavioral performance and task-related neural responses were dynamically changed during SD (Muto et al., 2016; Zhu et al., 2018, 2019). Further studies should investigate the dynamic changes in GMD during one night of SD. Fourth, the sample size in the present study was relatively small. Our findings should be verified in a larger sample. Finally, one night of SD can cause substantial deterioration of multiple types of cognitive performance. We only examined the relationship between GMD changes, PVT performance, and sleepiness. Further research should take other types of cognitive performance into consideration.
Conclusion
We identified increased GMD in the PFC and decreased GMV and CT in the TP after SD, and found a relationship between the △GMD in the MFG and the changes of sleepiness. These findings indicate that the brain gray matter structure is vulnerable to acute SD, and that alterations in GMD may be implicated in sleepiness after SD.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Research Ethics Committee of the Xijing Hospital of the Air Force Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JS, YX, HY, and WQ were guarantors of integrity of the entire study. JS, YZ, and WQ contributed to study concepts/study design. JS, YX, and HY contributed to data acquisition. JS, YC, and RZ contributed to data analysis/interpretation. JS, RZ, XY, and HD contributed to manuscript drafting or manuscript revision. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This work was financially supported by the Key Research and Development Program of Shaanxi (Program No. 2020ZDLSF04-05), the National Basic Research Program of China (Grant No. 2015CB856403), the Science and Technology Projects of Xi’an, China (Grant No. 201809170CX11JC12), the National Science Foundation of China (Grant No. 81901827), and the Fundamental Research Funds for the Central Universities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Sydney Koke, MFA, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this article.
Footnotes
References
Abel, T., Havekes, R., Saletin, J. M., and Walker, M. P. (2013). Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol. 23, R774–R788. doi: 10.1016/j.cub.2013.07.025
Acosta-Pena, E., Camacho-Abrego, I., Melgarejo-Gutierrez, M., Flores, G., Drucker-Colin, R., and Garcia-Garcia, F. (2015). Sleep deprivation induces differential morphological changes in the hippocampus and prefrontal cortex in young and old rats. Synapse 69, 15–25. doi: 10.1002/syn.21779
Akerstedt, T., Anund, A., Axelsson, J., and Kecklund, G. (2014). Subjective sleepiness is a sensitive indicator of insufficient sleep and impaired waking function. J. Sleep Res. 23, 240–252. doi: 10.1111/jsr.12158
Akerstedt, T., and Gillberg, M. (1990). Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 52, 29–37. doi: 10.3109/00207459008994241
Amorim, L., Magalhaes, R., Coelho, A., Moreira, P. S., Portugal-Nunes, C., Castanho, T. C., et al. (2018). Poor sleep quality associates with decreased functional and structural brain connectivity in normative aging: a MRI multimodal approach. Front. Aging Neurosci. 10:375. doi: 10.3389/fnagi.2018.00375
Areal, C. C., Warby, S. C., and Mongrain, V. (2017). Sleep loss and structural plasticity. Curr. Opin. Neurobiol. 44, 1–7. doi: 10.1016/j.conb.2016.12.010
Bellesi, M., Pfister-Genskow, M., Maret, S., Keles, S., Tononi, G., and Cirelli, C. (2013). Effects of sleep and wake on oligodendrocytes and their precursors. J. Neurosci. 33, 14288–14300. doi: 10.1523/Jneurosci.5102-12.2013
Bernardi, G., Cecchetti, L., Siclari, F., Buchmann, A., Yu, X. Q., Handjaras, G., et al. (2016). Sleep reverts changes in human gray and white matter caused by wake-dependent training. Neuroimage 129, 367–377. doi: 10.1016/j.neuroimage.2016.01.020
Bushey, D., Tononi, G., and Cirelli, C. (2011). Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 332, 1576–1581. doi: 10.1126/science.1202839
Chee, M. W. L., and Chuah, Y. M. L. (2007). Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc. Natl. Acad. Sci. U.S.A. 104, 9487–9492. doi: 10.1073/pnas.0610712104
Chen, Q., Yang, H., Zhou, N. Y., Sun, L., Bao, H. Q., Tan, L., et al. (2016). Inverse U-shaped association between sleep duration and semen quality: longitudinal observational study (MARHCS) in Chongqing, China. Sleep 39, 79–86. doi: 10.5665/sleep.5322
Cirelli, C. (2013). Sleep and synaptic changes. Curr. Opin. Neurobiol. 23, 841–846. doi: 10.1016/j.conb.2013.04.001
Cirelli, C., Gutierrez, C. M., and Tononi, G. (2004). Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron 41, 35–43. doi: 10.1016/S0896-6273(03)00814-6
Dang-Vu, T. T. (2013). Structural brain modifications in primary insomnia: Myth or Reality? Commentary on Joo et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep 36, 965–966. doi: 10.5665/sleep.2780
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Dickson, J. M., Weavers, H. M., Mitchell, N., Winter, E. M., Wilkinson, I. D., Van Beek, E. J. R., et al. (2005). The effects of dehydration on brain volume - Preliminary results. Int. J. Sports Med. 26, 481–485. doi: 10.1055/s-2004-821318
Diekelmann, S., and Born, J. (2010). The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126. doi: 10.1038/nrn2762
Duning, T., Kloska, S., Steinstrater, O., Kugel, H., Heindel, W., and Knecht, S. (2005). Dehydration confounds the assessment of brain atrophy. Neurology 64, 548–550. doi: 10.1212/01.Wnl.0000150542.16969.CC
Elvsashagen, T., Norbom, L. B., Pedersen, P. O., Quraishi, S. H., Bjornerud, A., Malt, U. F., et al. (2015). Widespread changes in white matter microstructure after a day of waking and sleep deprivation. PLoS One 10:e0127351. doi: 10.1371/journal.pone.0127351
Elvsashagen, T., Zak, N., Norbom, L. B., Pedersen, P. O., Quraishi, S. H., Bjornerud, A., et al. (2017). Evidence for cortical structural plasticity in humans after a day of waking and sleep deprivation. Neuroimage 156, 214–223. doi: 10.1016/j.neuroimage.2017.05.027
Frank, M. G., and Cantera, R. (2014). Sleep, clocks, and synaptic plasticity. Trends Neurosci. 37, 491–501. doi: 10.1016/j.tins.2014.06.005
Fuchs, T. A., Vaughn, C. B., Benedict, R. H. B., Weinstock-Guttman, B., Choudhery, S., Carolus, K., et al. (2019). Lower self-report fatigue in multiple sclerosis is associated with localized white matter tract disruption between amygdala, temporal pole, insula, and other connected structures. Mult. Scler. Relat. Disord. 27, 298–304. doi: 10.1016/j.msard.2018.11.005
Havekes, R., Park, A. J., Tudor, J. C., Luczak, V. G., Hansen, R. T., Ferri, S. L., et al. (2016). Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. eLife 5:e13424. doi: 10.7554/eLife.13424
Hinard, V., Mikhail, C., Pradervand, S., Curie, T., Houtkooper, R. H., Auwerx, J., et al. (2012). Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J. Neurosci. 32, 12506–12517. doi: 10.1523/Jneurosci.2306-12.2012
Horne, J. A. (1993). Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br. J. Psychiatry 162, 413–419. doi: 10.1192/bjp.162.3.413
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., and Smith, S. M. (2012). Fsl. Neuroimage 62, 782–790. doi: 10.1016/j.neuroimage.2011.09.015
Kempton, M. J., Ettinger, U., Schmechtig, A., Winter, E. M., Smith, L., McMorris, T., et al. (2009). Effects of acute dehydration on brain morphology in healthy humans. Hum. Brain Mapp. 30, 291–298. doi: 10.1002/hbm.20500
Killgore, W. D. S., Vanuk, J. R., Knight, S. A., Markowski, S. M., Pisner, D., Shane, B., et al. (2015). Daytime sleepiness is associated with altered resting thalamocortical connectivity. Neuroreport 26, 779–784. doi: 10.1097/Wnr.0000000000000418
Krause, A. J., Ben Simon, E., Mander, B. A., Greer, S. M., Saletin, J. M., Goldstein-Piekarski, A. N., et al. (2017). The sleep-deprived human brain. Nat. Rev. Neurosci. 18, 404–418. doi: 10.1038/nrn.2017.55
Langner, R., and Eickhoff, S. B. (2013). Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 139, 870–900. doi: 10.1037/a0030694
Lim, J. L., and Dinges, D. F. (2008). Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 1129, 305–322. doi: 10.1196/annals.1417.002
Liu, S., Liu, Q. L., Tabuchi, M., and Wu, M. N. (2016). Sleep drive is encoded by neural plastic changes in a dedicated circuit. Cell 165, 1347–1360. doi: 10.1016/j.cell.2016.04.013
Mongrain, V., Hernandez, S. A., Pradervand, S., Dorsaz, S., Curie, T., Hagiwara, G., et al. (2010). Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep 33, 1147–1157. doi: 10.1093/sleep/33.9.1147
Motomura, Y., Katsunuma, R., Yoshimura, M., and Mishima, K. (2017). Two Days’ sleep debt causes mood decline during resting state via diminished amygdala-prefrontal connectivity. Sleep 40:zsx133. doi: 10.1093/sleep/zsx133
Muto, V., Jaspar, M., Meyer, C., Kusse, C., Chellappa, S. L., Degueldre, C., et al. (2016). Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science 353, 687–690. doi: 10.1126/science.aad2993
Muto, V., Shaffii-Le Bourdiec, A., Matarazzo, L., Foret, A., Mascetti, L., Jaspar, M., et al. (2012). Influence of acute sleep loss on the neural correlates of alerting, orientating and executive attention components. J. Sleep Res. 21, 648–658. doi: 10.1111/j.1365-2869.2012.01020.x
Rasch, B., and Born, J. (2013). About Sleep’s role in memory. Physiol. Rev. 93, 681–766. doi: 10.1152/physrev.00032.2012
Reuter, M., and Fischl, B. (2011). Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 57, 19–21. doi: 10.1016/j.neuroimage.2011.02.076
Reuter, M., Rosas, H. D., and Fischl, B. (2010). Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181–1196. doi: 10.1016/j.neuroimage.2010.07.020
Reuter, M., Schmansky, N. J., Rosas, H. D., and Fischl, B. (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418. doi: 10.1016/j.neuroimage.2012.02.084
Santarnecchi, E., Del Bianco, C., Sicilia, I., Momi, D., Di Lorenzo, G., Ferrone, S., et al. (2018). Age of insomnia onset correlates with a reversal of default mode network and supplementary motor cortex connectivity. Neural Plast. 2018:3678534. doi: 10.1155/2018/3678534
Sepulcre, J., Masdeu, J. C., Goni, J., Arrondo, G., de Mendizabal, N. V., Bejarano, B., et al. (2009). Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult. Scler. J. 15, 337–344. doi: 10.1177/1352458508098373
Smith, S. M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. doi: 10.1002/hbm.10062
Streitburger, D. P., Moller, H. E., Tittgemeyer, M., Hund-Georgiadis, M., Schroeter, M. L., and Mueller, K. (2012). Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One 7:e44195. doi: 10.1371/journal.pone.0044195
Tahmasian, M., Noori, K., Samea, F., Zarei, M., Spiegelhalder, K., Eickhoff, S. B., et al. (2018). A lack of consistent brain alterations in insomnia disorder: an activation likelihood estimation meta-analysis. Sleep Med. Rev. 42, 111–118. doi: 10.1016/j.smrv.2018.07.004
Tanaka, M., Ishii, A., and Watanabe, Y. (2014). Neural effects of mental fatigue caused by continuous attention load: a magnetoencephalography study. Brain Res. 1561, 60–66. doi: 10.1016/j.brainres.2014.03.009
Thomas, C., and Baker, C. I. (2013). Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage 73, 225–236. doi: 10.1016/j.neuroimage.2012.03.069
Tononi, G., and Cirelli, C. (2006). Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62. doi: 10.1016/j.smrv.2005.05.002
Tononi, G., and Cirelli, C. (2014). Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34. doi: 10.1016/j.neuron.2013.12.025
Van Dongen, H. P. A., Maislin, G., Mullington, J. M., and Dinges, D. F. (2003). The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26, 117–126. doi: 10.1093/sleep/26.2.117
Walker, M. P., Stickgold, R., Alsop, D., Gaab, N., and Schlaug, G. (2005a). Sleep-dependent motor memory plasticity in the human brain. Neuroscience 133, 911–917. doi: 10.1016/j.neuroscience.2005.04.007
Walker, M. P., Stickgold, R., Jolesz, F. A., and Yoo, S. S. (2005b). The functional anatomy of sleep-dependent visual skill learning. Cereb. Cortex 15, 1666–1675. doi: 10.1093/cercor/bhi043
Whitney, P., Hinson, J. M., and Nusbaum, A. T. (2019). A dynamic attentional control framework for understanding sleep deprivation effects on cognition. Prog. Brain Res. 246, 111–126. doi: 10.1016/bs.pbr.2019.03.015
Xu, J. L., Zhu, Y. Q., Fu, C., Sun, J. B., Li, H. Q., Yang, X. J., et al. (2016). Frontal metabolic activity contributes to individual differences in vulnerability toward total sleep deprivation-induced changes in cognitive function. J. Sleep Res. 25, 169–180. doi: 10.1111/jsr.12354
Yang, F. N., Xu, S. H., Chai, Y., Basner, M., Dinges, D. F., and Rao, H. Y. (2018). Sleep deprivation enhances inter-stimulus interval effect on vigilant attention performance. Sleep 41:zsy189. doi: 10.1093/sleep/zsy189
Yeo, B. T. T., Tandi, J., and Chee, M. W. L. (2015). Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage 111, 147–158. doi: 10.1016/j.neuroimage.2015.02.018
Zhang, Y. Y., Brady, M., and Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57. doi: 10.1109/42.906424
Zhao, R., Zhang, X. X., Fei, N. B., Zhu, Y. Q., Sun, J. B., Liu, P., et al. (2019a). Decreased cortical and subcortical response to inhibition control after sleep deprivation. Brain Imaging Behav. 13, 638–650. doi: 10.1007/s11682-018-9868-2
Zhao, R., Zhang, X. X., Zhu, Y. Q., Fei, N. B., Sun, J. B., Liu, P. J., et al. (2019b). Disrupted resting-state functional connectivity in hippocampal subregions after sleep deprivation. Neuroscience 398, 37–54. doi: 10.1016/j.neuroscience.2018.11.049
Zhao, R., Zhang, X. X., Zhu, Y. Q., Fei, N. B., Sun, J. B., Liu, P., et al. (2018). Prediction of the effect of sleep deprivation on response inhibition via machine learning on structural magnetic resonance imaging data. Front. Hum. Neurosci. 12:276. doi: 10.3389/fnhum.2018.00276
Zhu, Y. A., Wang, L. X., Xi, Y. B., Dai, T., Fei, N. B., Liu, L., et al. (2017). White matter microstructural properties are related to inter-individual differences in cognitive instability after sleep deprivation. Neuroscience 365, 206–216. doi: 10.1016/j.neuroscience.2017.09.047
Zhu, Y. Q., Xi, Y. B., Fei, N. B., Liu, Y. C., Zhang, X. X., Liu, L., et al. (2018). Dynamics of cerebral responses to sustained attention performance during one night of sleep deprivation. J. Sleep Res. 27, 184–196. doi: 10.1111/jsr.12582
Keywords: sleep deprivation, gray matter density, voxel-based morphometry, gray matter volume, cortical thickness, psychomotor vigilance test, sleepiness
Citation: Sun J, Zhao R, Yang X, Deng H, Zhu Y, Chen Y, Yuan K, Xi Y, Yin H and Qin W (2020) Alteration of Brain Gray Matter Density After 24 h of Sleep Deprivation in Healthy Adults. Front. Neurosci. 14:754. doi: 10.3389/fnins.2020.00754
Received: 09 November 2019; Accepted: 26 June 2020;
Published: 11 August 2020.
Edited by:
Yuval Nir, Tel Aviv University, IsraelReviewed by:
Giulio Bernardi, IMT School for Advanced Studies Lucca, ItalyHengyi Rao, University of Pennsylvania, United States
Copyright © 2020 Sun, Zhao, Yang, Deng, Zhu, Chen, Yuan, Xi, Yin and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Qin, wqin@xidian.edu.cn
†These authors have contributed equally to this work
 Jinbo Sun1†
Jinbo Sun1† Yuanqiang Zhu
Yuanqiang Zhu Wei Qin
Wei Qin