A Single Nucleotide Polymorphism Within Molybdenum Cofactor Sulfurase Gene Is Associated With Neuropsychiatric Conditions
- 1Institute of Research and Development, Duy Tan University, Da Nang, Vietnam
- 2Department of Immunology, Ophthalmology and ENT, School of Medicine, Complutense University, Madrid, Spain
- 3Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Neurophysiology Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
- 5Urogenital Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Molybdenum cofactor sulfurase (MOCOS) is an enzyme participating in purine metabolism. The aim of current study was to evaluate the role of a single nucleotide polymorphism (SNP) in the coding gene (rs594445) in mood disorders and methamphetamine addiction.
Methods: This SNP was genotyped in 200 persons with methamphetamine addiction, 85 patients with bipolar disorder 1 (BP1), 78 patients with BP2, 33 patients with major depressive disorder (MDD) and 200 age-/sex-matched normal subjects using the tetra-primer amplification-refractory mutation system (ARMS)-PCR technique.
Results: The rs594445 was associated with methamphetamine addiction in co-dominant model [A/A vs C/C: OR (95% CI) = 0.466 (0.252–0.864), P-value = 0.014; C/A vs C/C: OR (95% CI) = 0.641 (0.418–0.981), P-value = 0.04]. This SNP was also associated with this trait in dominant model [OR (95% CI) = 0.591 (0.398–0.879), P-value = 0.009]. The A allele of rs594445 had a protective role against methamphetamine addiction [A vs C: OR (95% CI) = 0.645 (0.48–0.866), P-value = 0.004]. The rs594445 was associated with BP1 in co-dominant model [C/A vs C/C: OR (95% CI) = 0.423 (0.230–0.778), P-value = 0.005]. However, the associations were insignificant in other inheritance models.
Conclusion: Finally, there were no significant associations between the mentioned SNP and risk of BP2 or MDD in any inheritance model. The present project highlights the role rs594445 in two psychiatric conditions and implies the presence of common genetic factors for these disorders.
Introduction
Molybdenum cofactor sulfurase (MOCOS) being encoded by the MOCOS gene transfers a sulfur atom to the molybdenum cofactor of xanthine dehydrogenase (XDH) and aldehyde oxidase (AOX1) and activates these enzymes (Ichida et al., 2001; Taheri et al., 2020). Mutations in human MOCOS gene have been associated with classical xanthinuria type II (Ichida et al., 2001). More recently, MOCOS has been shown to be down-regulated in adult nasal olfactory stem cells of subjects with autism spectrum disorder (ASD) compared with controls. Moreover, functional studies have shown that dysregulation in expression of this gene leads to synaptic flaws as well as hypersensitivity to oxidative stress (Feron et al., 2016). MOCOS has been shown to be expressed in several regions of the developing and adult human brain. Moreover, studies in mouse brain tissues have verified its expression in the hippocampus, cortex, cerebellum and brainstem (Feron et al., 2016).
This gene has a number of single nucleotide polymorphisms (SNPs) among them is rs594445. A previous study has provided clues for functionality of this SNP. This study has shown that renal transplant recipients having minor allele of this SNP needed lower doses of Azathioprine for proficient immunosuppression following allograft transplantation (Kurzawski et al., 2012). Moreover, this SNP slightly protects patients with inflammatory bowel disease against occurrence of adverse drug reactions to Azathioprine (Smith et al., 2009).
Based on the proposed role for MOCOS in neurodevelopment and neurotransmission (Feron et al., 2016), we aimed to identify its role in two psychiatric conditions namely mood disorders and methamphetamine addiction. So, we genotyped a probable functional SNP within this gene in these patients and healthy subjects to find any difference in allelic frequencies between groups.
Materials and Methods
Enrolled Individuals
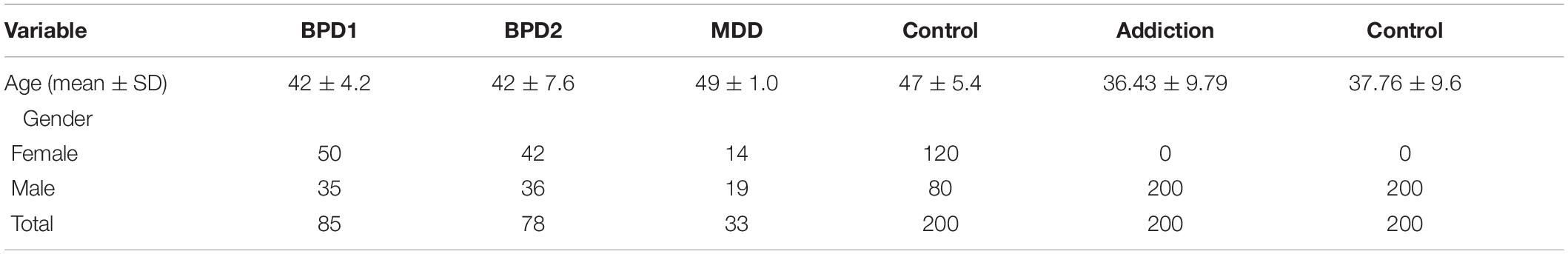
A total of 200 persons with methamphetamine addiction, 85 patients with BP1, 78 patients with BP2 and 33 patients with MDD joined the current project. Moreover, 200 age-/sex-matched normal subjects were chosen as controls. Patients with mood disorders were referred to Hospitals affiliated with Shahid Beheshti and Hamadan Universities of Medical Sciences during 2016–2019. Addicted persons were referred to Niaz and Atinegar Addiction Treatment Centers, Mashhad, Iran during 2017–2019. Both groups were assessed and diagnosed using the 5th edition of Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013). Individuals having any structural or metabolic brain disorders or medical conditions with psychiatric complications were excluded from the study. Persons with history of substance abuse were exempted from all groups excepting the “Addiction” group. Control subjects had no psychiatric conditions based on the results of a semi-structured interview. Written informed consent forms were signed by all enrolled persons. The study protocol was approved by the Ethics Committees of Shahid Beheshti Universities of Medical Sciences.
Genotyping
Table 1 shows the general features of the selected SNP.
Blood samples were obtained from all aces and controls in EDTA-containing tubes. DNA was extracted from these samples using conventional salting out method. The alleles of rs594445 SNP were defined in each study participants using the tetra-primer amplification-refractory mutation system (ARMS)-PCR technique. Primers were designed using the Primer1 software (Collins and Ke, 2012). Samples were prepared using the Taq DNA Polymerase Master Mix RED (Amplicon, Denmark). Table 2 displays the information about the primers sizes, annealing temperature and the anticipated product sizes. Amplifications were executed using the following PCR program: denaturing phase at 95°C for 7 min; 30 cycles at 95°C for 30 s, 59°C for 40 s, 72°C for 1 min and an ultimate extension phase at 72°C for 7 min.

Table 2. Nucleotide sequence of primers and PCR conditions (F: forward, R: reverse, i: inner, o: outer).
Statistical Assessments
All statistical tests were performed in R 3.2.2 software. Agreement/non-accordance with Hardy-Weinberg equilibrium (HWE) were evaluated in each study group using Chi-square test. Associations between the rs594445 allele/genotypes and mood disorders/methamphetamine addiction were evaluated in co-dominant, dominant, recessive and allelic models. Odds ratios (OR), 95% confidence intervals (95% CI) and P-values were computed to define the associations. P-values less than 0.05 were considered as significant.
Results
General Characteristics of Study Participants
General characteristics of study participants are listed in Table 3.
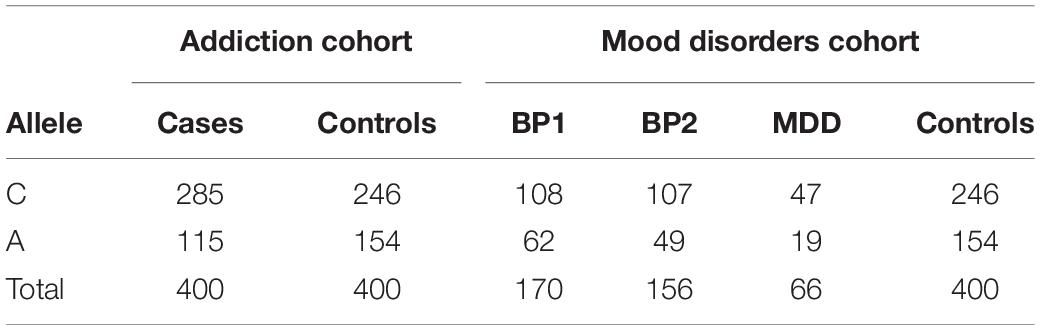
Frequencies of Different Alleles of rs594445
Table 4 shows the allele frequencies of the different alleles of rs594445 in patients and controls.
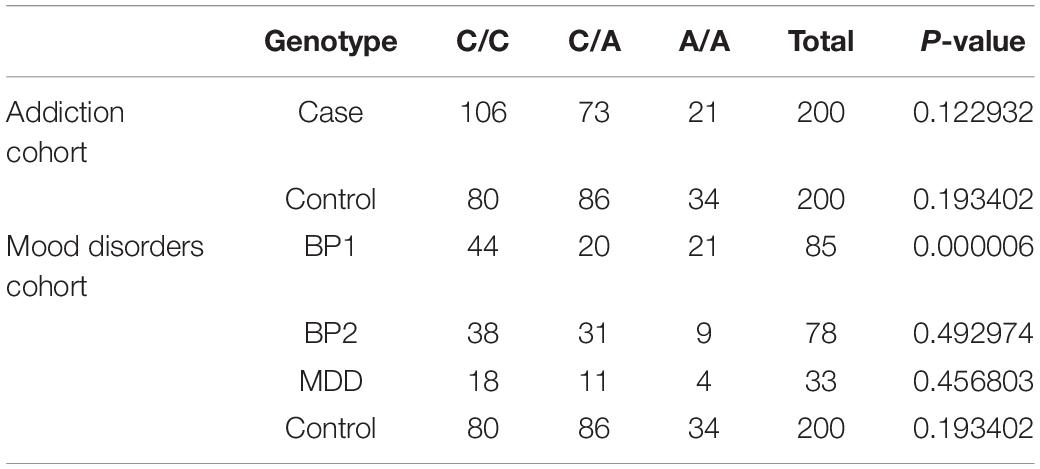
Genotype Frequencies
Genotype frequencies of the rs594445 were in agreement with the HWE assumption in all groups of psychiatric patients and healthy subjects except for BP1 group (Table 5).
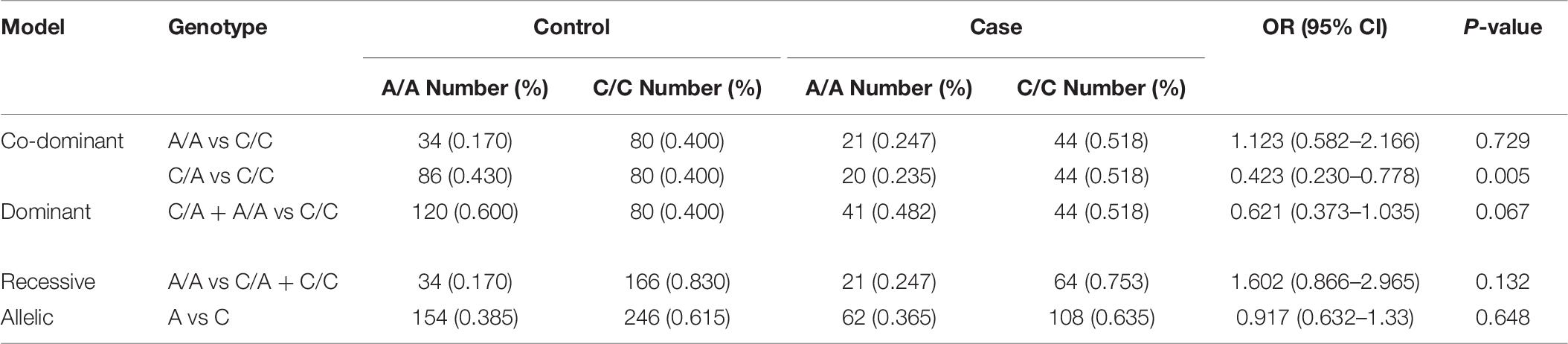
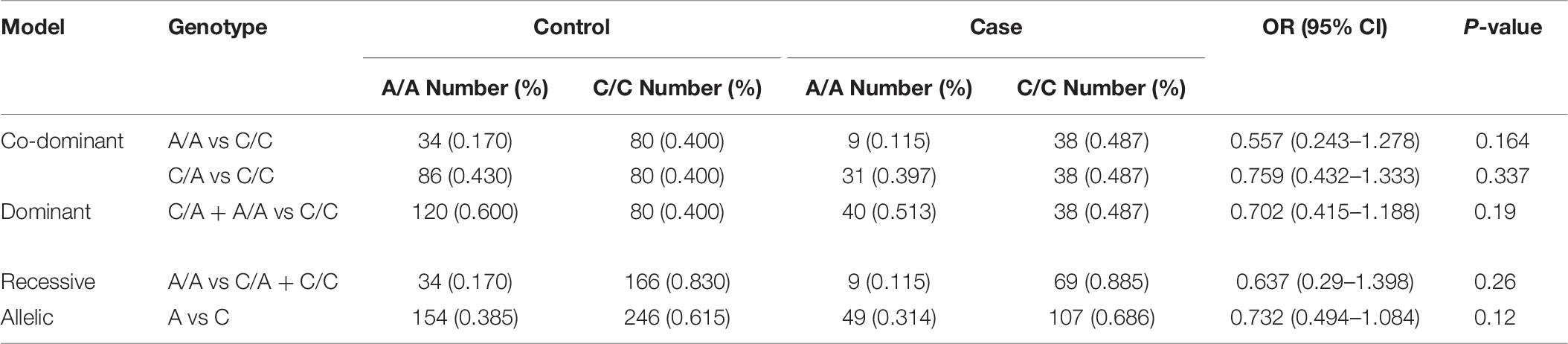
The rs594445 was associated with methamphetamine addiction in co-dominant model [A/A vs C/C: OR (95% CI) = 0.466 (0.252–0.864], P-value = 0.014; C/A vs C/C: OR (95% CI) = 0.641 (0.418–0.981), P-value = 0.04]. This SNP was also associated with this trait in dominant model [OR (95% CI) = 0.591 (0.398–0.879), P-value = 0.009]. The A allele of rs594445 had a protective role against methamphetamine addiction [A vs C: OR (95% CI) = 0.645 (0.48–0.866), P-value = 0.004]. Table 6 shows the detail analysis of associations between methamphetamine addiction and rs594445 SNP in four inheritance models.
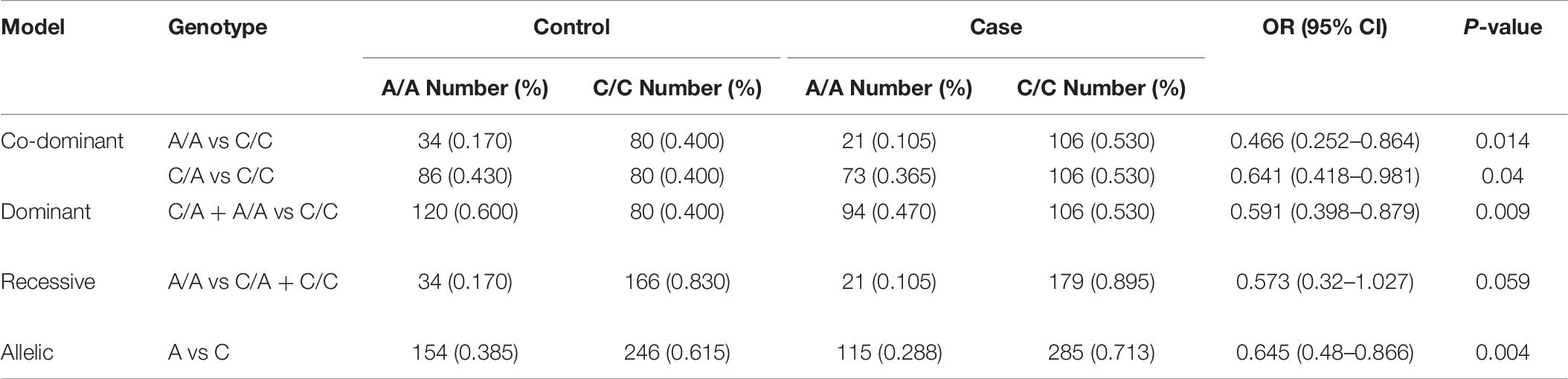
Table 6. Associations between methamphetamine addiction and rs594445 SNP in four inheritance models.
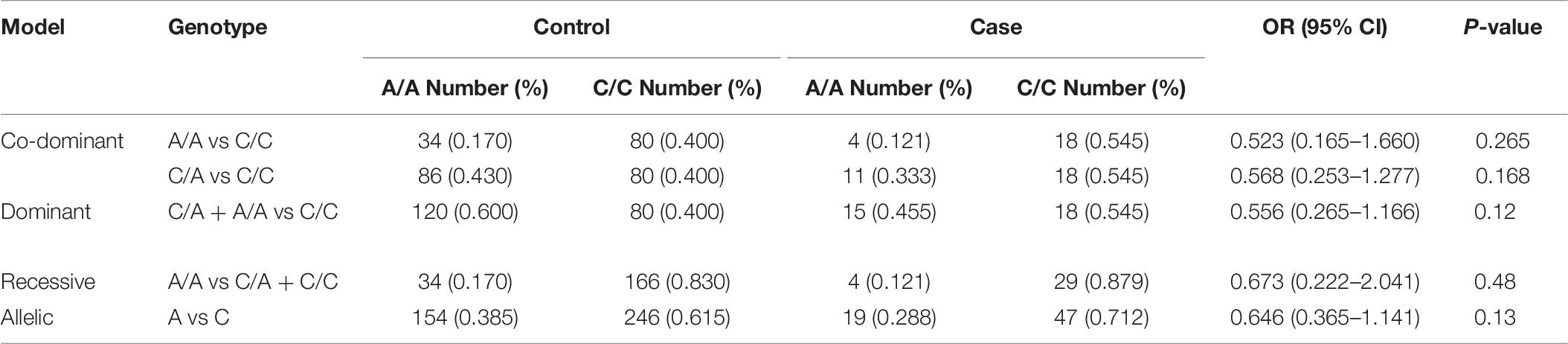
The rs594445 was associated with BP1 in co-dominant model [C/A vs C/C: OR (95% CI) = 0.423 (0.230–0.778), P-value = 0.005]. However, the associations were insignificant in other inheritance models (Table 7).
Finally, there were no significant associations between the mentioned SNP and risk of BP2 or MDD in any inheritance model (Tables 8, 9).
Discussion
In the present research project, we evaluated associations between rs594445 and mood disorders as well as methamphetamine addiction. This missense polymorphism may change enzyme activity of MOCOS and participate in the pathophysiology of human disorders. Although most of previous studies assessed its contribution in purine metabolism (Ichida et al., 2001), more recent studies reported expression of this gene in brain tissues and its participation in a neuropsychiatric condition namely ASD (Feron et al., 2016).
The rs594445 was associated with methamphetamine addiction in co-dominant, dominant and allelic models. Notably, the minor allele of rs594445 had a protective role against methamphetamine addiction. This allele might change MOCOS activity, and result in slower thiopurine metabolism (Kurzawski et al., 2012). However, the relevance of this altered enzymatic activity with methamphetamine addiction needs to be clarified. A previous study has shown changes in tryptophan and purine metabolism in cocaine-dependent individuals compared with controls (Patkar et al., 2009). Another study has indicated the effects of methamphetamine on the systemic metabolism in animal subjects (Zheng et al., 2014). The differences in metabolic pathways between carriers of different alleles of the rs594445 SNP might result in accumulation of specific metabolites following methamphetamine abuse leading to aversion. The similar mechanism has been explained for genetic factors that determine alcohol dependence. For instance SNPs within gene coding alcohol and aldehyde dehydrogenases may determine alcohol-dependence risk vs aversive response (Mayfield et al., 2008). However, such mechanisms have not been clarified for other substances which are subjects of addiction or abuse.
Besides, the rs594445 was associated with BP1 but not with BP2. The association between disturbed purine metabolism and mania has been acknowledged about one century ago (Kraepelin, 1921). Moreover, higher purinergic turnover has been suggested as a possible contributing factor in the pathogenesis of mania (Machado-Vieira et al., 2002). However, the reason for different pattern of association between BP1 and BP2 in our study is not clear. Future studies are needed to replicate these results in higher sample sizes of BP 1 and BP 2 patients and assess any possible difference.
Finally, there were no significant associations between the mentioned SNP and risk of BP2 or MDD in any inheritance model. This was an unexpected result as a previous study reported altered purine metabolism in MDD patients (Ali-Sisto et al., 2016). The mentioned study has shown decreased levels of inosine and guanosine, and elevated concentrations of xanthine in MDD patients compared with controls (Ali-Sisto et al., 2016). Future studies are needed to point out the specific genetic defect or alteration in purine metabolism pathway in these patients.
The present project highlights the role rs594445 in two psychiatric conditions and implies the presence of common genetic factors for these disorders. The underlying mechanism might be related to involvement of MOCOS in neurotransmission in developing and adult brain or its participation in regulation of purine metabolism. Our study has some limitations. First, we did not perform functional assays to appraise the effect of the nucleotide substitution on enzyme activity. Moreover, the sample size in BP2 and MDD subgroups were small. Therefore, the observed lack of association between rs594445 and these conditions may be due to small sample size.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The study protocol was approved by the Ethics Committees of Shahid Beheshti Universities of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AS and FN performed the experiment. AK and MD analyzed the data. MT and SG-F wrote the draft and revised it. All authors contributed to the article and approved the submitted version.
Funding
The current study was supported by a grant from Shahid Beheshti University of Medical Sciences (grant number: 21566).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, AS, declared a shared affiliation, with several of the authors, to the handling editor at the time of review.
References
Ali-Sisto, T., Tolmunen, T., Toffol, E., Viinamäki, H., Mäntyselkä, P., and Valkonen-Korhonen, M. (2016). Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology 70, 25–32. doi: 10.1016/j.psyneuen.2016.04.017
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders. BMC Med. 17, 133–137.
Collins, A., and Ke, X. (2012). Primer1: primer design web service for tetra-primer ARMS-PCR. Open Bioinform. J. 6, 55–58. doi: 10.2174/1875036201206010055
Feron, F., Gepner, B., Lacassagne, E., Stephan, D., Mesnage, B., Blanchard, M. P., et al. (2016). Olfactory stem cells reveal MOCOS as a new player in autism spectrum disorders. Mol. Psychiatry 21, 1215–1224.
Ichida, K., Matsumura, T., Sakuma, R., Hosoya, T., and Nishino, T. (2001). Mutation of human molybdenum cofactor sulfurase gene is responsible for classical xanthinuria type II. Biochem. Biophys. Res. Commun. 282, 1194–1200. doi: 10.1006/bbrc.2001.4719
Kraepelin, E. (1921). Manic depressive insanity and paranoia. J. Nerv. Ment. Dis. 53:350. doi: 10.1097/00005053-192104000-00057
Kurzawski, M., Dziewanowski, K., Safranow, K., and Drozdzik, M. (2012). Polymorphism of genes involved in purine metabolism (XDH, AOX1, MOCOS) in kidney transplant recipients receiving azathioprine. Ther. Drug Monit. 34, 266–274. doi: 10.1097/ftd.0b013e31824aa681
Machado-Vieira, R., Lara, D., Souza, D., and Kapczinski, F. (2002). Purinergic dysfunction in mania: an integrative model. Med. Hypothes. 58, 297–304. doi: 10.1054/mehy.2001.1543
Mayfield, R. D., Harris, R. A., and Schuckit, M. A. (2008). Genetic factors influencing alcohol dependence. Br. J. Pharmacol. 154, 275–287. doi: 10.1038/bjp.2008.88
Patkar, A. A., Rozen, S., Mannelli, P., Matson, W., Pae, C. U., Krishnan, K. R., et al. (2009). Alterations in tryptophan and purine metabolism in cocaine addiction: a metabolomic study. Psychopharmacology. 206, 479–489. doi: 10.1007/s00213-009-1625-1
Smith, M. A., Marinaki, A. M., Arenas, M., Shobowale-Bakre, M., Lewis, C. M., Ansari, A., et al. (2009). Novel pharmacogenetic markers for treatment outcome in azathioprine-treated inflammatory bowel disease. Aliment. Pharmacol. Ther. 30, 375–384. doi: 10.1111/j.1365-2036.2009.04057.x
Taheri, M., Noroozi, R., Aghaei, K., Omrani, M. D., and Ghafouri-Fard, S. (2020). The rs594445 in MOCOS gene is associated with risk of autism spectrum disorder. Metab. Brain Dis. 35, 497–501. doi: 10.1007/s11011-019-00524-y
Keywords: molybdenum cofactor sulfurase, rs594445, methamphetamine addiction, bipolar disorder, major depressive disorder
Citation: Safa A, Davood Omrani M, Nicknafs F, Komaki A, Taheri M and Ghafouri-Fard S (2020) A Single Nucleotide Polymorphism Within Molybdenum Cofactor Sulfurase Gene Is Associated With Neuropsychiatric Conditions. Front. Mol. Biosci. 7:540375. doi: 10.3389/fmolb.2020.540375
Received: 05 March 2020; Accepted: 25 August 2020;
Published: 24 September 2020.
Edited by:
Mario Hiroyuki Hirata, University of São Paulo, BrazilReviewed by:
Ana Cláudia Coelho, University of Trás-os-Montes and Alto Douro, PortugalArezou Sayad, Shahid Beheshti University of Medical Sciences, Iran
Rezvan Noroozi, Jagiellonian University, Poland
Copyright © 2020 Safa, Davood Omrani, Nicknafs, Komaki, Taheri and Ghafouri-Fard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, mohammad_823@yahoo.com; Soudeh Ghafouri-Fard, s.ghafourifard@sbmu.ac.ir
 Amin Safa1,2
Amin Safa1,2  Mohammad Taheri
Mohammad Taheri