Resonance Raman Optical Activity Spectroscopy in Probing Structural Changes Invisible to Circular Dichroism Spectroscopy: A Study on Truncated Vitamin B12 Derivatives
Abstract
:1. Introduction
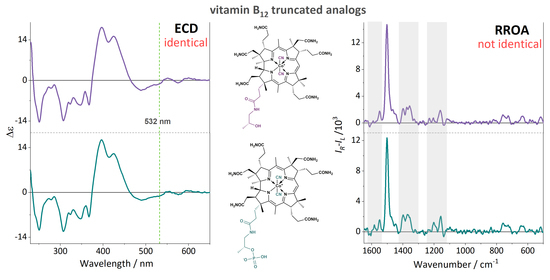
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Spectroscopic Measurements (UV-Vis/ECD, Raman/ROA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giedyk, M.; Jackowska, A.; Równicki, M.; Kolanowska, M.; Trylska, J.; Gryko, D. Vitamin B12 transports modified RNA into E. coli and S. typhimurium cells. Chem. Commum. 2019, 55, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Clardy, S.M.; Allis, D.G.; Fairchild, T.J.; Doyle, R.P. Vitamin B12 in drug delivery: Breaking through the barriers to a B12 bioconjugate pharmaceutical. Expert Opin. Drug Deliv. 2011, 8, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Braselmann, E.; Wierzba, A.J.; Polaski, J.T.; Chromiśnki, M.; Holmes, Z.E.; Hung, S.T.; Batan, D.; Wheeler, J.R.; Parker, R.; Jimenez, R.; et al. A multicolor riboswitch-based platform for imaging of RNA in live mammalian cells. Nat. Chem. Biol. 2018, 641, 343–372. [Google Scholar] [CrossRef]
- Zelder, F.; Sonnay, M.; Prieto, L. Antivitamins for Medicinal Applications. ChemBioChem 2015, 16, 1264–1278. [Google Scholar] [CrossRef]
- Zhou, K.; Oetterli, R.M.; Brandl, H.; Lyatuu, F.E.; Buckel, W.; Zelder, F. Chemistry and Bioactivity of an Artificial Adenosylpeptide B12 Cofactor. ChemBioChem 2012, 13, 2052–2055. [Google Scholar] [CrossRef] [Green Version]
- Giedyk, M.; Goliszewska, K.; Gryko, D. Vitamin B12 catalysed reactions. Chem. Soc. Rev. 2015, 44, 3391–3404. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Piao, Y.; Yamashita, M.; Kawaraichi, N.; Asegawa, R.; Ono, H.; Murooka, Y. Production of vitamin B12 in genetically engineered Propionibacterium freudenreichii. J. Biosci. Bioeng. 2004, 98, 167–173. [Google Scholar] [CrossRef]
- Thibaut, D.; Blanche, F.; Cameron, B.; Crouzet, J.; Debussche, L.; Rémy, E.; Vuilhorgne, M. Vitamin B12 Biosynthesis in Pseudomonas denitrificans. Vitam. B12 B12-Proteins 1998, 63–79. [Google Scholar] [CrossRef]
- Kim, J.; Gherasim, C.; Banerjee, R. Decyanation of vitamin B12 by a trafficking chaperone. Proc. Natl. Acad. Sci. USA 2008, 105, 14551–14554. [Google Scholar] [CrossRef] [Green Version]
- Gruber, K.; Puffer, B.; Kräutler, B. Vitamin B12-derivatives-enzyme cofactors and ligands of proteins and nucleic acids. Chem. Soc. Rev. 2011, 40, 4346–4363. [Google Scholar] [PubMed]
- Brown, K.L. Chemistry and Enzymology of Vitamin B12. Chem. Rev. 2005, 105, 2075–2150. [Google Scholar] [PubMed]
- Marsh, E.N.G.; Drennan, C.L. Adenosylcobalamin-dependent isomerases: New insights into structure and mechanism. Curr. Opin. Chem. Biol. 2001, 5, 499–505. [Google Scholar]
- Gerfen, G.J.; Licht, S.; Willems, J.P.; Hoffman, B.M.; Stubbe, J. Electron Paramagnetic Resonance Investigations of a Kinetically Competent Intermediate Formed in Ribonucleotide Reduction: Evidence for a Thiyl Radical-Cob(II)alamin Interaction. J. Am. Chem. Soc. 1996, 118, 8192–8197. [Google Scholar]
- Bridwell-Rabb, J.; Drennan, C.L. Vitamin B12 in the spotlight again. Curr. Opin. Chem. Biol. 2017, 37, 63–70. [Google Scholar]
- Proinsias, K.; Karczewski, M.; Zieleniewska, A.; Gryko, D. Microwave-Assisted Cobinamide Synthesis. J. Org. Chem. 2014, 79, 7752–7757. [Google Scholar]
- Zou, X.; Evans, D.R.; Brown, K.L. Efficient and Convenient Method for Axial Nucleotide Removal from Vitamin B12 and Its Derivatives. Inorg. Chem. 1995, 34, 1634–1635. [Google Scholar]
- Hassan, S.; Jackowska, A.; Gryko, D. Truncated vitamin B12 derivative with the phosphate group retained. J. Porphyr. Phthalocyanines 2019, 23, 554–560. [Google Scholar]
- Lee, J.; Mahon, S.B.; Mukai, D.; Burney, T.; Katebian, B.S.; Chan, A.; Bebarta, V.S.; Yoon, D.; Boss, G.R.; Brenner, M. The Vitamin B12 Analog Cobinamide Is an Effective Antidote for Oral Cyanide Poisoning. J. Med. Toxicol. 2016, 12, 370–379. [Google Scholar]
- Dereven’kov, I.A.; Salnikov, D.S.; Makarov, S.V.; Surducan, M.; Silaghi-Dumitrescu, R.; Boss, G.R. Comparative study of reaction of cobalamin and cobinamide with thiocyanate. J. Inorg. Biochem. 2013, 125, 32–39. [Google Scholar]
- Broderick, K.E.; Potluri, P.; Zhuang, S.; Scheffler, I.E.; Sharma, V.S.; Pilz, R.B.; Boss, G.R. Cyanide Detoxification by the Cobalamin Precursor Cobinamide. Exp. Biol. Med. 2006, 231, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Banerjee, R. Role of the Dimethylbenzimidazole Tail in the Reaction Catalyzed by Coenzyme B12-Dependent Methylmalonyl-CoA Mutase. Biochemistry 1999, 38, 15287–15294. [Google Scholar] [CrossRef] [PubMed]
- Andruniow, T.; Jaworska, M.; Lodowski, P.; Zgierski, M.Z.; Dreos, R.; Randaccio, L.; Kozlowski, P.M. Time-dependent density functional theory study of cobalt corrinoids: Electronically excited states of methylcobalamin. J. Chem. Phys. 2008, 129, 85101. [Google Scholar] [CrossRef] [PubMed]
- Reig, A.J.; Conrad, K.S.; Brunold, T.C. Combined Spectroscopic/Computational Studies of Vitamin B12 Precursors: Geometric and Electronic Structures of Cobinamides. Inorg. Chem. 2012, 51, 2867–2879. [Google Scholar] [CrossRef] [Green Version]
- Stich, T.A.; Buan, N.R.; Brunold, T.C. Spectroscopic and Computational Studies of Co2+ Corrinoids: Spectral and Electronic Properties of the Biologically Relevant Base-On and Base-Off Forms of Co2+ Cobalamin. J. Am. Chem. Soc. 2004, 126, 9735–9749. [Google Scholar] [CrossRef]
- Stich, T.A.; Buan, N.R.; Escalante-Semerena, J.C.; Brunold, T.C. Spectroscopic and Computational Studies of the ATP:Corrinoid Adenosyltransferase (CobA) from Salmonella enterica: Insights into the Mechanism of Adenosylcobalamin Biosynthesis. J. Am. Chem. Soc. 2005, 127, 8710–8719. [Google Scholar]
- Park, K.; Brunold, T.C. Combined Spectroscopic and Computational Analysis of the Vibrational Properties of Vitamin B12 in its Co3+, Co2+, and Co1+ Oxidation States. J. Phys. Chem. B 2013, 117, 5397–5410. [Google Scholar] [CrossRef]
- Park, K.; Mera, P.E.; Escalante-Semerena, J.C.; Brunold, T.C. Resonance Raman spectroscopic study of the interaction between Co (II) rrinoids and the ATP:corrinoid adenosyltransferase PduO from Lactobacillus reuteri. J. Biol. Inorg. Chem. 2016, 21, 669–681. [Google Scholar] [CrossRef]
- Dong, S.; Padmakumar, R.; Maiti, N.; Banerjee, R.; Spiro, T.G. Resonance Raman Spectra Show That Coenzyme B12 Binding to Methylmalonyl-Coenzyme A Mutase Changes the Corrin Ring Conformation but Leaves the Co-C Bond Essentially Unaffected. J. Am. Chem. Soc. 1998, 120, 9947–9948. [Google Scholar]
- Puckett, J.M.; Mitchell, M.B.; Hirota, S.; Marzilli, L.G. Near-IR FT-Raman Spectroscopy of Methyl-B12 and Other Cobalamins and of Imidazole and Imidazolate Methylcobinamide Derivatives in Aqueous Solution. Inorg. Chem 1996, 35, 4656–4662. [Google Scholar]
- Andruniow, T.; Zgierski, M.Z.; Kozlowski, P.M. Vibrational Analysis of Methylcobalamin. J. Phys. Chem. A 2002, 106, 1365–1373. [Google Scholar] [CrossRef]
- Mayer, E.; Gardiner, D.J.; Hester, R.E. Resonance Raman spectra of vitamin B12 and dicyanocobalamin. BBA Gen. Subj. 1973, 297, 568–570. [Google Scholar] [CrossRef]
- Eschenmoser, A.J.; Scheffold, R.; Bertele, E.; Pesaro, M.; Gschwend, H. Synthetic corrin complexes. Proc. R. Soc. A 1965, 288, 306–323. [Google Scholar]
- Kieninger, C.; Baker, J.A.; Podewitz, M.; Wurst, K.; Jockusch, S.; Lawrence, A.D.; Deery, E.; Gruber, K.; Liedl, K.R.; Warren, M.J.; et al. Zinc Substitution of Cobalt in Vitamin B12: Zincobyric acid and Zincobalamin as Luminescent Structural B12-Mimics. Angew. Chem. 2019, 58, 14568–14572. [Google Scholar]
- Bonnett, R. The chemistry of the vitamin B12 group. Chem. Rev. 1963, 63, 573–605. [Google Scholar]
- Bonnett, R.; Godfrey, J.M.; Math, V.B.; Scopes, P.M.; Thomas, R.N. Circular Dichroism of some Vitamin B12 Derivatives. J. Chem. Soc. Perkin Trans. I 1973, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Firth, R.A.; Hill, H.A.O.; Pratt, J.M.; Williams, R.J.P.; Jackson, W.R. The Circular Dichroism and Absorption Spectra of Some Vitamin B12 Derivatives. Biochemistry 1967, 6, 2178–2189. [Google Scholar]
- Liptak, M.D.; Brunold, T.C. Spectroscopic and Computational Studies of Co1+ Cobalamin: Spectral and Electronic Properties of the “Superreduced” B12 Cofactor. J. Am. Chem. Soc. 2006, 128, 9144–9156. [Google Scholar] [CrossRef]
- Wagner, F. Reactions of the cyano and alkyl cobalamins. Proc. R. Soc. Lond. 1965, 288, 344–347. [Google Scholar]
- Stich, T.A.; Brooks, A.J.; Buan, N.R.; Brunold, T.C. Spectroscopic and Computational Studies of Co3+-Corrinoids: Spectral and Electronic Properties of the B12 Cofactors and Biologically Relevant Precursors. J. Am. Chem. Soc. 2003, 125, 5897–5914. [Google Scholar] [CrossRef] [PubMed]
- Machalska, E.; Zajac, G.; Gruca, A.; Zobi, F.; Baranska, M.; Kaczor, A. Resonance Raman Optical Activity Shows Unusual Structural Sensitivity for Systems in Resonance with Multiple Excited States: Vitamin B12 Case. J. Phys. Chem. Lett. 2020, 11, 5037–5043. [Google Scholar] [CrossRef]
- Nafie, L.A. Theory of resonance Raman optical activity: The single electronic state limit. Chem. Phys. 1996, 205, 309–322. [Google Scholar] [CrossRef]
- Merten, C.; Li, H.; Nafie, L.A. Simultaneous Resonance Raman Optical Activity Involving Two Electronic States. J. Phys. Chem. A 2012, 116, 7329–7336. [Google Scholar] [CrossRef] [PubMed]
- Luber, S.; Neugebauer, J.; Reiher, M. Enhancement and de-enhancement effects in vibrational resonance Raman optical activity. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef] [PubMed]
- Zajac, G.; Kaczor, A.; Chruszcz-Lipska, K.; Dobrowolski, J.C.; Baranska, M. Bisignate resonance Raman optical activity: A pseudo breakdown of the single electronic state model of RROA? J. Raman Spectrosc. 2014, 45, 859–862. [Google Scholar] [CrossRef]
- Zajac, G.; Kaczor, A.; Buda, S.; Młynarski, J.; Frelek, J.; Dobrowolski, J.C.; Baranska, M. Prediction of ROA and ECD Related to Conformational Changes of Astaxanthin Enantiomers. J. Phys. Chem. B 2015, 119, 12193–12201. [Google Scholar] [CrossRef]
- Bogaerts, J.; Johannessen, C. On/off resonance Raman optical activity of human serum transferrin. J. Raman Spectrosc. 2019, 50, 641–646. [Google Scholar] [CrossRef]
- Firth, R.A.; Hill, H.A.O.; Mann, B.E.; Pratt, J.M.; Thorp, R.G.; Williams, R.J.P. The chemistry of vitamin B12. Part IX. Evidence for five-co-ordinate cobalt (III) complexes. J. Chem. Soc. A 1968, 2419–2428. [Google Scholar] [CrossRef]
- Day, P. A theory of the optical properties of vitamin B12 and its derivatives. Chim. Acta 1967, 7, 328–341. [Google Scholar] [CrossRef]
- Perlman, D.; Toohey, J.I. Cobalt-free Corrinoids as Vitamin B12 Antagonists. Nature 1966, 212, 300–301. [Google Scholar] [CrossRef]
- Brunold, T.C. Combined spectroscopic/computational studies of metal centers in proteins and cofactors: Application to coenzyme B12. CHIMIA Int. J. Chem. 2004, 58, 186–193. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Raman/cm−1 | ROA/cm−1 | Assignments |
|---|---|---|
| 1602 | - | C=C str (14-15), C=N str (16-24) |
| 1577 | 1578 (+) | C=C str (5-6; 10-11), C-H bend (10), C=N str (16-24; 4-21) |
| 1543 | 1546 (+) | C=C str (5-6; 10-11; 14-15), C=N str (9-22; 11-23; 16-24; 4-21) |
| 1501 | 1501 (+) | C=C str (5-6; 9-10; 14-15), C=N str (4-21; 11-23; 16-24; B2-B1; B2-B3), C-H bend (10; B2), CH3 bend asym, CH2 bend (scissoring) |
| 1350 | 1344 (+) | CH2 wagg, CH bend |
| 1228 | 1228 (+) | CH2 wagg, CH bend, NH bend, CH3 rock |
| 1205 | 1205 (+) | C-C str (corrin ring), CH2 twist, CH bend, CH3 rock |
| 1168 | 1168 (+) | C-N str (6-22; 14-23), C-C str (corrin ring), CH2 twist, CH bend, CH3 rock |
| 1105s | 1109 (−) | C-N str (6-22; 14-23), NH2 rock, CH2 twist, CH bend |
| 890 | 895s (+) 877 (+) | CH3 and CH2 rock, C-C str (7-37; 37-38; 8-9;6-7; 8-41; 9-10; 12-46), Co-N str (22; 23), C-C str (1-2; 1-19; 1-20; 3-4; 4-5; 5-35; 6-7; 7-8; 7-36; 31-32), C-N str (1-21) |
| 732 | 732 (+) | CH3 and CH2 rock, corrin ring tors and bend, NH2 twist, CCC bend, CC, CN tors, benzimidazole C-C str |
| 696 | 695 (−) | CH2 rock, NH2 twist, corrin ring tors and bend, CCC bend, CC, CN tors |
| 636 | 635 (+) | NH2 twist, corrin ring tors and bend |
| 584 | 585 (+) | NH2 twist |
| 518 | 518 (+) | corrin ring tors and bend, NH2 twist, CCC bend, CC, CN tors |
| 496 | 497 (−) | Co-C≡N bend, C≡N twist, corrin ring tors and bend, benzimidazole ring breathing, Co-N bend (B3), O-H bend (7R) |
| 427 | 429 (+) | Co-C≡N bend, corrin ring tors and bend, CCN, CNC bend, CC, CN tors, C≡N twist, O-H bend (7R) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machalska, E.; Zajac, G.; Halat, M.; Wierzba, A.J.; Gryko, D.; Baranska, M. Resonance Raman Optical Activity Spectroscopy in Probing Structural Changes Invisible to Circular Dichroism Spectroscopy: A Study on Truncated Vitamin B12 Derivatives. Molecules 2020, 25, 4386. https://doi.org/10.3390/molecules25194386
Machalska E, Zajac G, Halat M, Wierzba AJ, Gryko D, Baranska M. Resonance Raman Optical Activity Spectroscopy in Probing Structural Changes Invisible to Circular Dichroism Spectroscopy: A Study on Truncated Vitamin B12 Derivatives. Molecules. 2020; 25(19):4386. https://doi.org/10.3390/molecules25194386
Chicago/Turabian StyleMachalska, Ewa, Grzegorz Zajac, Monika Halat, Aleksandra J. Wierzba, Dorota Gryko, and Malgorzata Baranska. 2020. "Resonance Raman Optical Activity Spectroscopy in Probing Structural Changes Invisible to Circular Dichroism Spectroscopy: A Study on Truncated Vitamin B12 Derivatives" Molecules 25, no. 19: 4386. https://doi.org/10.3390/molecules25194386