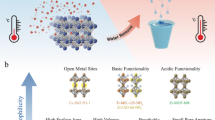
Abstract
Amine absorbents that efficiently absorb and desorb CO2 in response to small temperature changes are desired for CO2 separation from concentrated and dilute gases. Thermoresponsive hydrogel films consisting of amine-containing microgel particles (GPs), which capture CO2 at a low temperature (30 °C) and desorb it upon mild heating (75 °C), are attractive for capturing CO2 from postcombustion gases containing 10% CO2 (10 kPa). However, little information has been reported about thermoresponsive GPs for CO2 separation from gas mixtures with low concentrations of CO2. Herein, we describe the effect of the pKa of ammonium ions in GPs on the amount of CO2 desorption upon heating at 75 °C, which was investigated at various CO2 concentrations. The efficiency of CO2 desorption (mol-desorbed CO2/mol-amine) depends on the pKa and pKa shift (ΔpKa) of the ammonium ions in the range of 30‒75 °C. Computational predictions also indicated that the pKa values and ΔpKa are both important for reversible CO2 absorption. A guideline for designing thermoresponsive amine absorbents for various applications including direct air capture and carbon recycling in closed spaces, such as space stations and submarines, is provided.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bui M, Adjiman CS, Bardow A, Anthony EJ, Boston A, Brown S, et al. Carbon capture and storage (CCS): the way forward. Energy Environ Sci. 2018;11:1062–176.
Vega F, Baena-Moreno FM, Fernández LMG, Portillo E, Navarrete B, Zhang ZE. Current status of CO2 chemical absorption research applied to CCS: towards full deployment at industrial scale. Appl Energy. 2020;260:114313.
Wang M, Joel AS, Ramshaw C, Eimer D, Musa NM. Process intensification for post-combustion CO2 capture with chemical absorption a critical review. Appl Energy. 2015;158:275–91.
Wang. M, Lawal A, Stephenson P, Sidders J, Ramshaw C. Post-combustion CO2 capture with chemical absorption: a state-of-the-art review. Chem Eng Res Des. 2011;89:1609–24.
Leeson D, Fennell P, Shah N, Petit C, Mac Dowell N. A techno‐economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries. Int J Greenh Gas Control. 2017;61:71–84.
Rochelle GT. Amine scrubbing for CO2 capture. Science. 2009;325:1652–4.
Du Y, Yuan Y, Rochelle GT. Capacity and absorption rate of tertiary amine and hindered amines blended with piperazine for CO2 capture. Chem Eng Sci. 2016;155:397–404.
Gao H, Wu Z, Liu H, Luo X, Liang Z. Experimental studies on the effect of tertiary amine promoters in aqueous monoethanolamine (MEA) solutions on the absorption/stripping performances in post-combustion CO2 capture. Energy Fuels. 2017;31:13883.
Bernhardsen IM, Krokvik IRT, Perinu C, Pinto DDD, Jens KJ, Knuutila HK. Influence of pKa on solvent performance of MAPA promoted tertiary amines. Int J Greenh Gas Control. 2018;68:68–76.
Hwang SJ, Lee M, Kim H, Lee KS. Cyclic CO2 absorption capacity of aqueous single and blended amine solvents. J Ind Eng Chem. 2018;65:95–103.
Lin P-H, Wong DSH. Carbon dioxide capture and regeneration with amine/alcohol/water blends. Int J Greenh Gas Control. 2014;26:69–75.
Novek E, Shaulsky E, Fishman Z, Pfefferle L, Elimelech M. Low-temperature carbon capture using aqueous ammonia and organic solvents. Environ Sci Technol Lett. 2016;3:291–6.
Lai QH, Kong LL, Gong WB, Russell AG, Fan M. Low-energy-consumption and environmentally friendly CO2 capture via blending alcohols into amine solution. Appl Energy. 2019;254:113696.
Park Y, Lin KYA, Park AHA, Petit C. Recent advances in anhydrous solvents for CO2 capture: ionic liquids, switchable solvents, and nanoparticle organic hybrid materials. Front Energy Res. 2015;3:42.
Liu F, Jing G, Zhou X, Lv B, Zhou Z. Performance and mechanisms of triethylene tetramine (TETA) and 2-amino-2-methyl-1-propanol (AMP) in aqueous and non-aqueous solutions for CO2 capture. ACS Sustain Chem Eng. 2018;6:1352–61.
Yu. YS, Lu HF, Zhang TT, Zhang ZX, Wang GX, Rudolph V. Determining the performance of an efficient non-aqueous CO2 capture process at desorption temperature below 373 K. Ind Eng Chem Res. 2013;52:12622–34.
Zhang X, Liu H, Liang Z, Idem R, Tontiwachwuthikul P, Jaber Al-Marri M, et al. Reducing energy consumption of CO2 desorption in CO2-loaded aqueous amine solution using Al2O3/HZSM-5 bifunctional catalysts. Appl Energy. 2018;229:562–76.
Zhang X, Huang Y, Gao H, Luo X, Liang Z, Tontiwachwuthikul P. Zeolite catalyst-aided tri-solvent blend amine regeneration: an alternative pathway to reduce the energy consumption in amine-based CO2 capture process. Appl Energy. 2019;240:827–41.
Zhang X, Huang Y, Yang J, Gao H, Huang Y, Luo X, et al. Amine-based CO2 capture aided by acid-basic bifunctional catalyst: advancement of amine regeneration using metal modified MCM-41. Chem Eng J. 2020;383:123077.
Qi G, Fu L, Giannelis EP. Sponges with covalently tethered amines for high-efficiency carbon capture. Nat Commun. 2014;5:5796.
Heydari-gorji A, Yang Y, Sayari A. Effect of the pore length on CO2 adsorption over amine-modified mesoporous silicas. Energy Fuels. 2011;25:4206–10.
Chowdhury FA, Yamada H, Higashii T, Goto K, Onoda M. CO2 capture by tertiary amine absorbents: a performance comparison study. Ind Eng Chem Res. 2013;52:8323–31.
Xiao M, Liu H, Idem R, Tontiwachwuthikul P, Liang Z. A study of structure-activity relationships of commercial tertiary amines for post-combustion CO2 capture. Appl Energy. 2016;184:219–29.
Singto S, Supap T, Idem R, Tontiwachwuthikul P, Tantayanon S, Al-Marri MJ, et al. Synthesis of new amines for enhanced carbon dioxide (CO2) capture performance: the effect of chemical structure on equilibrium solubility, cyclic capacity, kinetics of absorption and regeneration, and heats of absorption and regeneration. Sep Purif Technol. 2016;167:97–107.
Narimani M, Amjad-Iranagh S, Modarress H. Performance of tertiary amines as the absorbents for CO2 capture: quantum mechanics and molecular dynamics. Stud J Nat Gas Sci Eng. 2017;47:154–66.
Numaguchi R, Fujiki J, Yamada H, Firoz, Chowdhury A, Kida K, et al. Development of post-combustion CO2 capture system using amine-impregnated solid sorbent. Energy Procedia. 2017;114:2304–12.
Yamada H, Chowdhury FA, Fujiki J, Yogo K. Enhancement mechanism of the CO2 adsorption–desorption efficiency of silica-supported tetraethylenepentamine by chemical modification of amino groups. ACS Sustain Chem Eng. 2019;7:9574–81.
Hoshino Y, Imamura K, Yue M, Inoue G, Miura Y. Reversible absorption of CO2 triggered by phase transition of amine-containing micro- and nanogel particles. J Am Chem Soc. 2012;134:18177–80.
Yue M, Hoshino Y, Ohshiro Y, Imamura K, Miura Y. Temperature-responsive microgel films as reversible carbon dioxide absorbents in wet environment. Angew Chem Int Ed. 2014;53:2654–7.
Gao J, Liu Y, Hoshino Y, Inoue G. Amine-containing nanogel particles supported on porous carriers for enhanced carbon dioxide capture. Appl Energy. 2019;253:113567.
Yue M, Hoshino Y, Miura Y. Design rationale of thermally responsive microgel particle films that reversibly absorb large amounts of CO2: fine tuning the pKa of ammonium ions in the particles. Chem Sci. 2015;6:6112–23.
Hoshino Y, Ohashi RC, Miura Y. Rational design of synthetic nanoparticles with a large reversible shift of acid dissociation constants: proton imprinting in stimuli responsive nanogel particles. Adv Mater. 2014;26:3718–23.
Hoshino Y, Miyoshi T, Nakamoto M, Miura Y. Wide-Range pKa tuning of proton imprinted nanoparticles for reversible protonation of target molecules via thermal stimuli. J Mater Chem B. 2017;5:9204–10.
Socolow R, Desmond M, Aines R, Blackstock J, Bolland O, Kaarsberg T, et al. Direct air capture of CO2 with chemicals: a technology assessment for the APS Panel on Public Affairs. Am Phys Soc. 2011.
Kiani A, Jiang K, Feron P. Techno-economic assessment for CO2 capture from air using a conventional liquid-based absorption process. Front Energy Res. 2020;8:92.
Sanz-Perez ES, Murdock CR, Didas SA, Jones CW. Direct capture of CO2 from ambient air. Chem Rev. 2016;116:11840–76.
Satyapal S, Filburn T, Trela J, Strange J. Performance and properties of a solid amine sorbent for carbon dioxide removal in space life support applications. Energy Fuels. 2001;15:250–5.
Zhao C, Guo Y, Li C, Lu S. Removal of low concentration CO2 at ambient temperature using several potassium-based sorbents. Appl Energy. 2014;124:241–7.
Huang Z, Chen ZB, Ren NQ, Hu DX, Zheng DH, Zhang ZP. A novel application of the SAWD-Sabatier-SPE integrated system for CO2 removal and O2 regeneration in submarine cabins during prolonged voyages. J Zhejiang Univ Sci A. 2009;10:1642–50.
Field CB, Mach KJ. Rightsizing carbon dioxide removal. Science. 2017;356:706–7.
Gabrielsen J, Michelsen ML, Stenby EH, Kontogeoegis GM. A model for estimating CO2 solubility in aqueous alkanolamines. Ind Eng Chem Res. 2005;44:3348–54.
McCann N, Maeder M, Attalla M. Simulation of enthalpy and capacity of CO2 absorption by aqueous amine systems. Ind Eng Chem Res. 2008;47:2002–9.
Didas SA, Kulkarni AR, Sholl DS, Jones CW. Role of amine structure on carbon dioxide adsorption from ultradilute gas streams such as ambient air. ChemSusChem. 2012;5:2058–64.
Aronu UE, Gondal S, Hessen ET, Haug-Warberg T, Hartono A, Hoff KA, et al. Solubility of CO2 in 15, 30, 45 and 60 mass% MEA from 40 to 120 °C and model representation using the extended UNIQUAC framework. Chem Eng Sci. 2011;66:6393–406.
Hwang SJ, Kim J, Kim H, Lee KS. Solubility of carbon dioxide in aqueous solutions of three secondary amines: 2-(Butylamino)Ethanol, 2-(Isopropylamino)Ethanol, and 2-(Ethylamino)Ethanol secondary alkanolamine solutions. J Chem Eng Data. 2017;62:2428–35.
Liu H, Chan C, Tontiwachwuthikul P, Idem R. Analysis of CO2 equilibrium solubility of seven tertiary amine solvents using thermodynamic and ANN models. Fuel. 2019;249:61–72.
Donaldson TL, Nguyen YN. Carbon dioxide reaction kinetics and transport in aqueous amine membranes Ind. Eng Chem Fundam. 1980;19:260–6.
Carroll JJ, Slupsky JD, Mather AE. The solubility of carbon dioxide in water at low pressure. J Phys Chem Ref Data. 1991;20:1201–9.
Marshall WL, Franck EU. Ion product of water substance, 0–1,000°C, 1–10,000 bars new international formulation and its background. J Phys Chem Ref Data. 1981;10:295–304.
Plummer LN, Busenberg E. The solubilities of calcite, aragonite, and vaterite in CO2-H2O solutions between 0 °C and 90 °C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim Cosmochim Acta. 1982;46:1011–40.
Katchalsky A, Spitnik P. Potentiometric titrations of polymethacrylic acid. J Polym Sci. 1947;2:432–46.
Kleinen J, Richtering W. Polyelectrolyte microgels based on Poly-N-isopropylacrylamide: influence of charge density on microgel properties, binding of poly-diallyldimethylammonium chloride, and properties of polyelectrolyte complexes. Colloid Polym Sci. 2011;289:739–49.
Acknowledgements
This research was supported by JSPS KAKENHI Grant Number JP15H05486, Japan; MEXT Innovative Areas of “Fusion Materials”, Grant Number 25107726, Japan; and JST-ALCA Grant Number JPMJAL1403, Japan and Japan Association for Chemical Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Honda, R., Hamasaki, A., Miura, Y. et al. Thermoresponsive CO2 absorbent for various CO2 concentrations: tuning the pKa of ammonium ions for effective carbon capture. Polym J 53, 157–167 (2021). https://doi.org/10.1038/s41428-020-00407-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00407-5
This article is cited by
-
Hydrogel particles for CO2 capture
Polymer Journal (2024)
-
Controlling the shell structure of hard core/hydrogel shell microspheres
Colloid and Polymer Science (2022)
-
Special issue: CO2: capture of, utilization of, and degradation into
Polymer Journal (2021)