Abstract
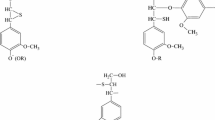
Results of the microwave-assisted catalytic pyrolysis of lignin have been described. It has been shown that, in a medium of an inert gas (argon) and a hydrogen–argon mixture, the following maximum liquid yield is achieved: 33 and 36% for iron-containing catalyst samples and 45% for nickel-containing catalysts. The gas chromatography–mass spectrometry (GC/MS) method has been used to determine the qualitative composition of the liquid product fraction, which is mostly represented by phenol and anisol, i.e., derivatives of the monomeric units of lignin (coumaryl and coniferyl alcohols). Probable sites of interaction of the functional groups of the lignin surface with nanosized particles of nickel- and iron-containing catalysts have been studied by IR spectroscopy.




Similar content being viewed by others
REFERENCES
R. M. Rowell, R. Pettersen, J. S. Han, et al., Handbook of Wood Chemistry and Wood Composites, Ed. by R. M. Rowell (CRC, Boca Raton, 2005), p. 9.
W.-J. Liu, H. Jiang, and H.-Q. Yu, Green Chem. 17, 4888 (2015).
M. L. Rabinovich, in Proceedings of the 2nd Nordic Wood Biorefinery Conference (Helsinki, 2009), p. 111.
C. N. Hamelink, G. van Hooijdonk, and A. P. C. Faaij, Biomass Bioenergy 28, 384 (2005).
D. M. Alonso, C. G. Wettstein, and J. A. Dumesic, Chem. Soc. Rev. 41, 8075 (2012).
C.-H. Zhou, X. Xia, C.-X. Lin, et al., Chem. Soc. Rev. 40, 5588 (2011).
D. M. Alonso, J. Q. Bond, and J. A. Dumesic, Green Chem. 12, 1493 (2010).
J. Zakzeski, P. C. Bruijnincx, A. L. Jongerius, and B. M. Weckhuysen, Chem. Rev. 110, 3552 (2010).
M. Brebu and C. Vasile, Cellul. Chem. Technol. 44, 353 (2010).
P. K. Swain, L. M. Das, and S. N. Naik, Renew. Sust. Energy Rev. 15, 4917 (2011).
P. McKendry, Bioresour. Technol. 83, 47 (2002).
M. Hamaguchi, M. Cardoso, and E. Vakkilainen, Energies 5, 2288 (2012).
J. Zakzeski and B. Weckhuysen, ChemSusChem 4, 369 (2011).
H. D. Willauer, J. G. Huddleston, M. Li, and R. D. Rogers, J. Chromatogr., B: Biomed. Appl. 743, 127 (2000).
S. R. Collinson and W. Thielemans, Coord. Chem. Rev. 254, 1854 (2010).
R. M. Ravenelle, J. R. Copeland, W. G. Kim, et al., ACS Catal. 1, 552 (2011).
R. M. Ravenelle, J. R. Copeland, A. H. van Pelt, et al., Top. Catal. 55, 162 (2012).
R. Y. Nsimba, C. A. Mullen, N. M. West, and A. A. Boateng, ACS Sust. Chem. Eng. 1, 260 (2013).
P. R. Patwardhan, R. C. Brown, and B. H. Shanks, ChemSusChem 4, 1629 (2011).
R. Lanza, D. D. Nogare, and P. Canu, Ind. Eng. Chem. Res. 48, 1391 (2009).
X. L. Zhuang, H. X. Zhang, J. Z. Yang, and H. Y. Qi, Bioresour. Technol 79, 63 (2001).
D. Lv, M. Xu, X. Liu, et al., Fuel Process. Technol. 91, 903 (2010).
X. H. Hao, L. J. Guo, X. Mao, et al., Int. J. Hydrogen Energy 28, 55 (2003).
A. J. Byrd, K. K. Pant, and R. B. Gupta, Ind. Eng. Chem. Res. 46, 3574 (2007).
A. J. Byrd, K. K. Pant, and R. B. Gupta, Energy Fuels 21, 3541 (2007).
W. Yunpu, D. Leilei, F. Liangliang, et al., J. Anal. Appl. Pyrolys. 119, 104 (2016).
J. Xu, J. Jiang, C. Hse, and T. F. Shupe, Green Chem. 14, 2821 (2012).
J. Xie, J. Qi, C. Hse, and T. F. Shupe, J. For. Res. 26, 261 (2015).
J. Xu, J. Jiang, C. Hse, and T. F. Shupe, Green Chem. 14, 2821 (2012).
J. Xie, J. Qi, C. Hse, and T. F. Shupe, J. For. Res. 26, 261 (2015).
H. G. Kim and Y. Park, Ind. Eng. Chem. Res. 52, 10 059 (2013).
J. Y. Kim, J. H. Lee, J. Park, et al., J. Anal. Appl. Pyrolys. 114, 273 (2015).
Q. Bu, H. Lei, L. Wang, et al., Bioresour. Technol. 162, 142 (2014).
L. Fan, P. Chen, Y. Zhang, et al., Bioresour. Technol. 225, 199 (2017).
M. V. Tsodikov, G. I. Konstantinov, A. V. Chistyakov, et al., Chem. Eng. J. 292, 315 (2016).
F. S. Wen, F. Zhang, and Z. Y. Liu, J. Phys. Chem. C 115 (29), 14 025 (2011).
G. V. Rodicheva, V. P. Orlovskii, N. M. Romanova, et al., Russ. J. Inorg. Chem. 41, 728 (1996).
M. V. Tsodikov, M. A. Perederii, M. S. Karaseva, et al., Nauk. Tekhnol., Nos. 6–7, 55 (2007).
M. V. Tsodikov, O. G. Ellert, S. A. Nikolaev, et al., J. Nanoparticle Res. 20 (3), 86 (2018).
M. V. Tsodikov, M. A. Perederii, M. S. Karaseva, et al., Nauk. Tekhnol., Nos. 6–7, 55 (2007).
I. G. Sudakova and N. B. Rudenko, Zh. Sib. Fed. Univ., Khim. 201, 499 (2015).
Thermochemical conversion of biomass to liquid fuels and chemicals, Ed. by M. Crocker (Royal Society of Chemistry, Cambridge, 2010).
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-33-01096mol_a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Arapova, O.V., Chistyakov, A.V., Palankoev, T.A. et al. Microwave-Assisted Lignin Conversion to Liquid Products in the Presence of Iron and Nickel. Pet. Chem. 60, 1019–1025 (2020). https://doi.org/10.1134/S0965544120090029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544120090029