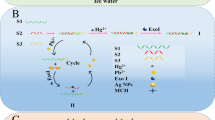
Abstract
A highly sensitive and selective electrochemical biosensor for Pb2+ with a dual-amplification strategy is proposed. The first amplification step was realized by the cycle of Pb2+ and 8–17 DNAzyme (S2), and the hybridization chain reaction (HCR) triggered by S1 further amplified the electrochemical signal. Fe3O4@Au NPs, as a multifunctional magnetic carrier, is not only manifested in the construction of a magnetically controlled electrochemical response interface, but also has significant contribution in the purifying system, reducing interference, increasing the specific surface area, and the DNA loading. The magnetic nanocomposites were characterized by TEM as spheres with particle size of around 39 nm. When there was no Pb2+, long double-strand DNA (dsDNA) is formed on the surface of Fe3O4@Au NPs by the S1-triggered HCR; in the presence of Pb2+, S2 is activated and S1 on the surface of magnetic biocomposites (Fe3O4@Au NPs-S1) is continuously cleaved with the cycle of Pb2+ and S2, leading to a significant decrease of methylene blue (MB) absorbed on dsDNA. Such reverse dual-signal amplification strategy effectively increased the current difference and improved the sensitivity of the proposed sensor. The electrochemical signal of MB was obtained by differential pulse voltammetry (DPV) with preconcentration, showing a linear response toward Pb2+ ranging from 50 pM to 1 μM with a detection limit of 15 pM. The proposed method has feasible applications in detecting other heavy metal ions based on other metal-dependent DNAzyme.

Graphical Abstract







Similar content being viewed by others
References
Cai L-M, Wang Q-S, Luo J, Chen L-G, Zhu R-L, Wang S, Tang C-H (2019) Heavy metal contamination and health risk assessment for children near a large Cu-smelter in Central China. Sci Total Environ 650:725–733
Peng T, O'Connor D, Zhao B, Jin Y, Zhang Y, Tian L, Zheng N, Li X, Hou D (2019) Spatial distribution of lead contamination in soil and equipment dust at children's playgrounds in Beijing, China. Environ Pollut 245:363–370
Llop S, Aguinagalde X, Vioque J, Ibarluzea J, Guxens M, Casas M, Murcia M, Ruiz M, Amurrio A, Rebagliato M, Marina LS, Fernandez-Somoano A, Tardon A, Ballester F (2011) Prenatal exposure to lead in Spain: cord blood levels and associated factors. Sci Total Environ 409(11):2298–2305
He Q, Miller EW, Wong AP, Chang CJ (2006) A selective fluorescent sensor for detecting lead in living cells. J Am Chem Soc 128(29):9316–9317
Lu Y, Li X, Wang G, Tang W (2013) A highly sensitive and selective optical sensor for Pb2+ by using conjugated polymers and label-free oligonucleotides. Biosens Bioelectron 39(1):231–235
World Health Organization, Geneva (2011) WHO guidelines for drinking-water quality, 4th edn. Who, Geneva, pp 504–505
Bings NH, Bogaerts A, Broekaert JAC (2006) Atomic spectroscopy. Anal Chem 78/12:3917–3946
Serpell CJ, Rutte RN, Geraki K, Pach E, Martincic M, Kierkowicz M, De Munari S, Wals K, Raj R, Ballesteros B, Tobias G, Anthony DC, Davis BG (2016) Carbon nanotubes allow capture of krypton, barium and lead for multichannel biological X-ray fluorescence imaging. Nat Commun 7:13118
Luo R, Xiaohong S, Xu W, Zhang S, Zhuo X, Dong M (2017) Determination of arsenic and lead in single hair strands by laser ablation inductively coupled plasma mass spectrometry. Sci Rep 7:3421
Liu S, Wei W, Sun X, Wang L (2016) Ultrasensitive electrochemical DNAzyme sensor for lead ion based on cleavage-induced template-independent polymerization and alkaline phosphatase amplification. Biosens Bioelectron 83:33–38
Zhang Y, Xiao S, Li H, Liu H, Pang P, Wang H, Wu Z, Yang W (2016) A Pb2+-ion electrochemical biosensor based on single-stranded DNAzyme catalytic beacon. Sensors Actuators B Chem 222:1083–1089
Wen Z-B, Liang W-B, Zhuo Y, Xiong C-Y, Zheng Y-N, Yuan R, Chai Y-Q (2017) An efficient target–intermediate recycling amplification strategy for ultrasensitive fluorescence assay of intracellular lead ions. Chem Commun 53(54):7525–7528
Yang Z, Wu H, Yi X, Tang J, Yun W, Han W, Chen X (2019) A universal converting strategy based on target-induced DNA nanoprobe conformational change for lead (II) ion assay. Biosens Bioelectron 144:111679
Zhang L, Deng H, Yuan R, Yuan Y (2019) Electrochemical lead(II) biosensor by using an ion-dependent split DNAzyme and a template-free DNA extension reaction for signal amplification. Microchim Acta 186:709
Hai H, Yang F, Li J (2014) Highly sensitive electrochemiluminescence “turn-on” aptamer sensor for lead(II) ion based on the formation of a G-quadruplex on a graphene and gold nanoparticles modified electrode. Microchim Acta 181(9):893–901
Song X, Wang Y, Liu S, Zhang X, Wang J, Wang H, Zhang F, Yu J, Huang J (2019) A triply amplified electrochemical lead(II) sensor by using a DNAzyme and via formation of a DNA-gold nanoparticle network induced by a catalytic hairpin assembly. Mikrochim Acta 186/8:559
Zhu C, Zhu W, Xu L, Zhou X (2019) A label-free electrochemical aptasensor based on magnetic biocomposites with Pb2+-dependent DNAzyme for the detection of thrombin. Anal Chim Acta 1047:21–27
Chen D, Zhang M, Ma M, Hai H, Li J, Yang S (2019) A novel electrochemical DNA biosensor for transgenic soybean detection based on triple signal amplification. Anal Chim Acta 1078:24–31
Han Q, Shen X, Zhu W, Zhu C, Zhou X, Jiang H (2016) Magnetic sensing film based on Fe3O4@Au-GSH molecularly imprinted polymers for the electrochemical detection of estradiol. Biosens Bioelectron 79:180–186
Zhu W, Jiang G, Xu L, Li B, Cai Q, Jiang H, Zhou X (2015) Facile and controllable one-step fabrication of molecularly imprinted polymer membrane by magnetic field directed self-assembly for electrochemical sensing of glutathione. Anal Chim Acta 886:37–47
Pang Y, Wan N, Shi L, Wang C, Sun Z, Xiao R, Wang S (2019) Dual-recognition surface-enhanced Raman scattering(SERS)biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal Chim Acta 1077:288–296
Premaratne G, Dharmaratne AC, Al Mubarak ZH, Mohammadparast F, Andiappan M, Krishnan S (2019) Multiplexed surface plasmon imaging of serum biomolecules: Fe3O4@Au Core/shell nanoparticles with plasmonic simulation insights. Sens Actuators B: Chem 299:126956
Choi HMT, Beck VA, Pierce NA (2014) Next-generation in situ hybridization chain reaction: higher gain, lower cost, greater durability. ACS Nano 8/5:4284–4294
Zhou Q, Lin Y, Zhang K, Li M, Tang D (2018) Reduced graphene oxide/BiFeO3 nanohybrids-based signal-on photoelectrochemical sensing system for prostate-specific antigen detection coupling with magnetic microfluidic device. Biosens Bioelectron 101:146–152
Chu Y, Deng A-P, Wang W, Zhu J-J (2019) Concatenated catalytic hairpin assembly/hyperbranched hybridization chain reaction based enzyme-free signal amplification for the sensitive photoelectrochemical detection of human telomerase RNA. Anal Chem 91(5):3619–3627
Chen X, Huang J, Zhang S, Mo F, Shasha S, Li Y, Fang L, Deng J, Huang H, Luo Z, Zheng J (2019) Electrochemical biosensor for DNA methylation detection through hybridization chain-amplified reaction coupled with a tetrahedral DNA nanostructure. ACS Appl Mater Interfaces 11(4):3745–3752
Hou T, Li W, Liu X, Li F (2015) Label-free and enzyme-free homogeneous electrochemical biosensing strategy based on hybridization chain reaction: a facile, sensitive, and highly specific MicroRNA assay. Anal Chem 87(22):11368–11374
Rohs R, Sklenar H, Lavery R, Röder B (2000) Methylene blue binding to DNA with alternating GC base sequence: a modeling study. J Am Chem Soc 122(12):2860–2866
Drummond TG, Hill MG and Barton JK (2003) Electrochemical DNA sensors. Nat Biotechnol 21/10:1192–1199
Baker BR, Lai RY, Wood MCS, Doctor EH, Heeger AJ, Plaxco KW (2006) An electronic, Aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J Am Chem Soc 128(10):3138–3139
Chen J, Zhang J, Wang K, Lin X, Huang L, Chen G (2008) Electrochemical biosensor for detection of BCR/ABL fusion gene using locked nucleic acids on 4-aminobenzenesulfonic acid-modified glassy carbon electrode. Anal Chem 80(21):8028–8034
Xuan F, Luo X, Hsing I-M (2012) Ultrasensitive solution-phase electrochemical molecular beacon-based DNA detection with signal amplification by exonuclease III-assisted target recycling. Anal Chem 84(12):5216–5220
Wu J, Yang Z, Chen N, Zhu W, Hong J, Huang C, Zhou X (2015) Vanillin-molecularly targeted extraction of stir bar based on magnetic field induced self-assembly of multifunctional Fe3O4@polyaniline nanoparticles for detection of vanilla-flavor enhancers in infant milk powders. J Colloid Interface Sci 442:22–29
Zhang H, Ma L, Li P, Zheng J (2016) A novel electrochemical immunosensor based on nonenzymatic Ag@Au-Fe3O4 nanoelectrocatalyst for protein biomarker detection. Biosens Bioelectron 85:343–350
Shen X, Ge Z, Pang Y (2015) Conjugating folate on superparamagnetic Fe3O4@Au nanoparticles using click chemistry. J Solid State Chem 222:37–43
Funding
This work was supported by the National Natural Science Foundation of China (No. 81773681, 81572081, 21904069) and the Natural Science Foundation of Jiangsu Province (No. BK20190653). This work was also supported by Chinese Postdoctoral Science Foundation (No. 2019M661777, 2017M621789) and the Postdoctoral Science Foundation of Jiangsu (No. 2019K068, 2019Z176).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experiments were performed in compliance with the relevant laws and institutional guidelines.
Conflict of interest
The authors declare that they have no conflict interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1949 kb)
Rights and permissions
About this article
Cite this article
Weng, C., Li, X., Lu, Q. et al. A label-free electrochemical biosensor based on magnetic biocomposites with DNAzyme and hybridization chain reaction dual signal amplification for the determination of Pb2+. Microchim Acta 187, 575 (2020). https://doi.org/10.1007/s00604-020-04548-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04548-5