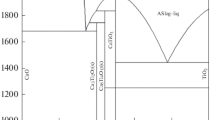
Substantiation of using a carbothermal method for processing flotation perovskite concentrate is presented. Results of X-ray structural studies of compacted specimens after two sintering stages are provided. It is shown that titanium carbide is formed in the first stage, and in the second stage calcium carbide is formed followed by dissociation into calcium metal and graphite. A dependence is obtained for the degree of transformation of titanium carbide on isothermal exposure duration and excess carbon in the initial charge. Dependence of degree of calcium extraction on isothermal exposure duration is demonstrated. It is shown that the temperature for the beginning of calcium carbidization at a given pressure is ≈ 1600°C. It is established that briquette compaction pressure has almost no effect on calcium extraction, while it affects briquette mechanical strength.
Thermodynamic calculations of equilibrium compositions of phases in contact involved in production processes are performed using HSC Chemistry application software. Results of differential-thermal and thermogravimetric analyses of a charge of a given composition of flotation perovskite and soot are analyzed. In order to study kinetic regularities, correlation coefficients of isothermal exposure duration and reaction rate constant are calculated using kinetic equations of Yander, Gistling–Brownstein, Zhuravlev–Lesokhin–Tempel’man, anti-Gistling, anti-Yander, and Tamman. Apparent activation energy for the first stage of the carbothermal process is calculated using a specific model taking account of results of experiments conducted at different temperatures (Ea = 311.48 kJ/mol). It is established that the dependence obtained for the degree of transformation on isothermal exposure duration is most adequately described by an anti-Yander model formal kinetics equation. Optimum production conditions are selected for the process on the basis of research results: briquette pressing pressure is 8.57 MPa; first stage temperature 1500°C and 1750°C in the second stage; heating rate 17°C/min and 9°C/min respectively. In this case exposure time is 120 min and 60 min in the first and second stages, and residual pressure is 50 Pa and 13.3·10–3 Pa respectively.











Similar content being viewed by others
References
V. G. Maiorov, A. I. Nikolaev, and V. K. Kopkov, “Separation of thorium (IV) during utilization of sulfuric acid solutions for breaking down perovskite,” Zh. Prikl. Khim., No. 5, 715–719 (2004).
G. F. Krusenko, D. G. Épov, and M. A. Medkov, “Comprehensive treatment of perovskite concentrate by fluoride technology,” Vestn. DVO RAN, No. 4, 113–117 92015).
O. A. Nikolaeva, “Prospects for development of production for titanium raw material of the Kola peninsula deposits,” Strategiya Razvit. Ékonomiki, 31–36 (2012).
“State and Use of Mineral Resources of the Russian Federation in 2016 and 2017,” in: State Paper (2018), pp. 203–214.
S. V. Sokolov, G. N. Nechelyustov, and I. G. Bystrov, “ Perovskite from titanium-magnetite perovskite ores of the Afrikanda deposit,” in: Earth Alkali Magnetism and Strategic Metal Bonded with Deposits, Alkali Magmatism of the Earth, Proc. XXXIII Internat. Conf. Inst. Geochem. and Analyt. Chem. im V. I. Vernadskogo RAN (GEOKhI RAN) (2016).
O. N. Budin, A. N. Kropachev, D. G. Arafonov, and V. V. Cherepov, “Study of carbothermal method for breaking down titanium raw material on the example of artificial synthesized perovskite,” Izv. Vuzov. Tsvet. Met., No. 5, 23–20 (2010); DOI: https://doi.org/10.17073/0021-3438-2018-5-23-30.
D. L. Motov, “Production solution for perovskite problem,” Tr. Fersman. Nauch. Sess. GI KNTs RAN, No. 7, 187–192 (2010).
Chlorination of Titanium Carbide; El. source URL: https://www.911metallurgist.com/chlorination-titanium-carbide/(access date 09.29.2019).
O. G. Parfenov and G. L. Pashkov, Titanium Contemporary Metallurgy Problems [in Russian], Izd. SO RAN, Novosibirsk (2008).
S. S. Korovin, G. V. Zimina, and A. M. Reznik, Rare and Dissipated Elements. Chemistry and Technology, Book 2, High SchoolTextbook [in Russian], MISiS, Moscow (1996).
A. N. Zelikman and V. G. Korshunov, Rare Metal Metallurgy: High School Textbook [in Russian], Metallurgiya, Moscow (1991).
Bin Li, Zhangfu Yuan, Wenbing Li, Cong Xu, “A new method for deoxidization and chlorination of refined ilmenite with high magnesia and calcia contents,” China Particuology, 2, No. 2, 84–88 (2004); DOI: https://doi.org/10.1016/S1672-2515(07)60029-3
Wensheng Zhang, Zhaowu Zhu, Chu Yong Cheng, “A literature review of titanium metallurgical processes,” Hydrometallurgy, 108, No. 3-4, 177–188 (2011); DOI: https://doi.org/10.1016/j.hydromet.2011.04.005
J. P. Bonsack and F. E. Schneider, “Entrained-flow chlorination of titaniferous slag to produce titanium tetrachloride,” Metallurgical and Materials Transactions B, 32, No. 3, 389–393 (2001); DOI:https://doi.org/10.1007/s11663-001-0022-x
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 64, No. 5, pp. 56–64, May, 2020.
Rights and permissions
About this article
Cite this article
Budin, O.N., Kropachev, A.N. & Сherepov, V.V. Study of Technology for Preparing Titanium Carbide and Calcium Metal from Perovskite Concentrate by a Carbothermal Method. Metallurgist 64, 446–459 (2020). https://doi.org/10.1007/s11015-020-01013-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-020-01013-9