Hair Growth Activity of Three Plants of the Polynesian Cosmetopoeia and Their Regulatory Effect on Dermal Papilla Cells
Abstract
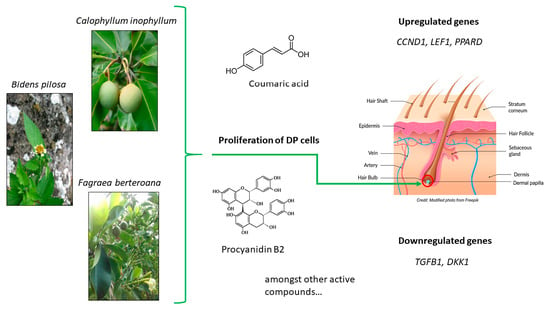
:1. Introduction
2. Results
2.1. Chemical Characterization of Plant Extracts
2.1.1. Colorimetric Assays
2.1.2. Characterization and Structural Determination of Compounds by Ultra Performance Liquid Chromatography UPLC-MS/MS
O-glycosyl Flavonoids
2.2. Biological Assays
2.2.1. Antioxidant (FRAP) and Anti-inflammatory (5-LOX) Activities of Extracts In Vitro
2.2.2. Proliferative Effect of Extracts and Fractions on Dermal Papilla Cells after 24 h and 48 h Treatment
2.3. Hair Growth Potential Mediated through Regulation of Hair Growth Factors in DPCs
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Determination of Polyphenol, Flavonoid, Anthocyanin and Saponin Contents
4.3. UHPLC-MS/MS Analysis
4.4. Ferric Reducing Antioxidant Power (FRAP) Assay
4.5. 5-Lipoxygenase (5-LOX) Assay
[O.D. sample]t = 0 min) × 100/([O.D. control]t = 1 min − [O.D. control]t = 0 min)).
4.6. Primary Culture of Human Hair Follicle Dermal Papilla Cells (HFDPCs)
4.7. Measurement of Cell Viability with the MTT Assay
4.8. RNA Isolation, Reverse Transcription and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ansel, J.-L.; Moretti, C.; Raharivelomanana, P.; Hano, C. Cosmetopoeia. Comptes Rendus Chim. 2016, 19, 1033–1034. [Google Scholar] [CrossRef]
- Girardi, C.; Butaud, J.F.; Ollier, C.; Ingert, N.; Weniger, B.; Raharivelomanana, P.; Moretti, C. Herbal medicine in the Marquesas Islands. J. Ethnopharmacol. 2015, 161, 200–213. [Google Scholar] [CrossRef]
- Jost, X.; Ansel, J.-L.; Lecellier, G.; Raharivelomanana, P.; Butaud, J.F. Ethnobotanical survey of cosmetic plants used in Marquesas Islands (French Polynesia). J. Ethnobiol. Ethnomed. 2016, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Pétard, P. Plantes utiles de Polynésie française et raau Tahiti; Editions Haere Po No Tahiti: Papeete, French Polynesia, 1986; ISBN 978-2-904171-06-2. [Google Scholar]
- Hughes, K.; Ho, R.; Butaud, J.-F.; Filaire, E.; Ranouille, E.; Berthon, J.-Y.; Raharivelomanana, P. A selection of eleven plants used as traditional Polynesian cosmetics and their development potential as anti-aging ingredients, hair growth promoters and whitening products. J. Ethnopharmacol. 2019, 245, 112159. [Google Scholar] [CrossRef] [PubMed]
- Handy, E.S.C. The Native Culture in the Marquesas; Bernice, P., Ed.; Bishop Museum: Honolulu, HI, USA, 1923. [Google Scholar]
- Whistler, W.A. Plants in Samoan Culture: The Ethnobotany of Samoa; Isle Botanica: Honolulu, HI, USA, 2000; ISBN 978-0-9645426-6-2. [Google Scholar]
- Brown, F.B.H. Flora of Southeastern Polynesia. III. Dicotyledons; Bernice, P., Ed.; Bishop Museum: Honolulu, HI, USA, 1935. [Google Scholar]
- Henry, T. Tahiti aux temps anciens; Publication de la Société des Océanistes n°1, Musée de l’homme: Paris, France, 1962. [Google Scholar]
- Zepernick, B. Arzneipflanzen der Polynesier: Von Bernhard Zepernick; Verlag von Dietrich Reimer: Berlin, Germany, 1972. [Google Scholar]
- Krauss, B.H. Ethnobotany of Hawaii (Unpublished Manuscript); Department of Botany, University of Hawaii: Mānoa, HI, USA, 1974. [Google Scholar]
- Dweck, A.C.; Meadows, T. Tamanu (Calophyllum inophyllum)—The African, Asian, Polynesian and Pacific Panacea. Int. J. Cosmet. Sci. 2002, 24, 341–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whistler, W.A. The Ethnobotany of Tongan Plants, Their Tongan Names and Their Uses; Bishop Museum Press: Honolulu, HI, USA, 1991; Vol. Bishop Museum Bulletin Botany 2. [Google Scholar]
- Whistler, W.A. Polynesian Herbal Medicine; National Tropical Botanical Garden: Kauai, HI, USA, 1992. [Google Scholar]
- McClatchey, W. The ethnopharmacopoeia of Rotuma. J. Ethnopharmacol. 1996, 50, 147–156. [Google Scholar] [CrossRef]
- Aitken, R.T. Ethnology of Tubuai; Bernice, P., Ed.; Bishop Museum: Honolulu, HI, USA, 1930; Volume 70. [Google Scholar]
- Whistler, W. Traditional and herbal medicine in the Cook islands. J. Ethnopharmacol. 1985, 13, 239–280. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Cantelli, M.; Masarà, A.; Annunziata, M.; Marasca, C.; Cacciapuoti, S. Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. Int. J. Women’s Dermatol. 2018, 4, 203–211. [Google Scholar] [CrossRef]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2002, 47, 377–385. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, O.; Chung, J.-H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Chi, W.; Wu, E.; Morgan, B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013, 140, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahoda, C.A.B.; Horne, K.A.; Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984, 311, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem and Progenitor Cells in Development and Regeneration of the Hair Follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laron, E.A.; Aamar, E.; Enshell-Seijffers, D. The Mesenchymal Niche of the Hair Follicle Induces Regeneration by Releasing Primed Progenitors from Inhibitory Effects of Quiescent Stem Cells. Cell Rep. 2018, 24, 909–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Ma, X.; Guo, H.; Deng, F.; Yang, J.; Li, Y. Wnt5a Suppresses β-catenin Signaling during Hair Follicle Regeneration. Int. J. Med. Sci. 2016, 13, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Wnt5a attenuates Wnt/β-catenin signalling in human dermal papilla cells. Exp. Dermatol. 2013, 22, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Mikels, A.J.; Nusse, R. Purified Wnt5a Protein Activates or Inhibits β-Catenin–TCF Signaling Depending on Receptor Context. PLoS Boil. 2006, 4, e115. [Google Scholar] [CrossRef]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.-J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Plikus, M.V. New activators and inhibitors in the hair cycle clock: Targeting stem cells’ state of competence. J. Investig. Dermatol. 2012, 132, 1321–1324. [Google Scholar] [CrossRef] [Green Version]
- Truong, V.-L.; Bak, M.-J.; Lee, C.; Jun, M.; Jeong, W.-S. Hair Regenerative Mechanisms of Red Ginseng Oil and Its Major Components in the Testosterone-Induced Delay of Anagen Entry in C57BL/6 Mice. Molecules 2017, 22, 1505. [Google Scholar] [CrossRef] [Green Version]
- Dastan, M.; Najafzadeh, N.; Abedelahi, A.; Sarvi, M.; Niapour, A. Human platelet lysate versus minoxidil stimulates hair growth by activating anagen promoting signaling pathways. Biomed. Pharmacother. 2016, 84, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- MassBank of North America (MoNA)—PubChem Data Source. Available online: https://pubchem.ncbi.nlm.nih.gov/source/22043 (accessed on 20 August 2020).
- Yamagaki, T.; Watanabe, T.; Tanaka, M.; Sugahara, K. Laser-Induced Hydrogen Radical Removal in UV MALDI-MS Allows for the Differentiation of Flavonoid Monoglycoside Isomers. J. Am. Soc. Mass Spectrom. 2013, 25, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Lim, C.; Lee, J.Y.; Im, K.R.; Yoon, K.-S.; Song, J.M. Visible-to-Near IR Quantum Dot-Based Hypermulticolor High-Content Screening of Herbal Medicines for the Efficacy Monitoring of Hair Growth Promotion and Hair Loss Inhibition. J. Biomol. Screen. 2013, 18, 462–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwack, M.H.; Kang, B.M.; Kim, M.-K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef]
- Boyera, N.; Galey, I.; Bernard, B. Biphasic Effects of Minoxidil on the Proliferation and Differentiation of Normal Human Keratinocytes. Ski. Pharmacol. Physiol. 1997, 10, 206–220. [Google Scholar] [CrossRef]
- Rendl, M.; Polak, L.; Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008, 22, 543–557. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. BioEssays 2005, 27, 247–261. [Google Scholar] [CrossRef]
- Kitagawa, T.; Matsuda, K.-I.; Inui, S.; Takenaka, H.; Katoh, N.; Itami, S.; Kishimoto, S.; Kawata, M. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J. Clin. Endocrinol. Metab. 2009, 94, 1288–1294. [Google Scholar] [CrossRef] [Green Version]
- Kafri, P.; E Hasenson, S.; Kanter, I.; Sheinberger, J.; Kinor, N.; Yunger, S.; Shav-Tal, Y. Quantifying β-catenin subcellular dynamics and cyclin D1 mRNA transcription during Wnt signaling in single living cells. eLife 2016, 5, e16748. [Google Scholar] [CrossRef]
- Inui, S.; Itami, S. Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J. Dermatol. Sci. 2011, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Itami, S. Androgen actions on the human hair follicle: Perspectives. Exp. Dermatol. 2012, 22, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dickkopf 1 Promotes Regression of Hair Follicles. J. Investig. Dermatol. 2012, 132, 1554–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.S.; Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Attenuation of Dickkopf 1-Induced Hair Growth Inhibition in Cultured Human Hair Follicles by Tianeptine. Ann. Dermatol. 2017, 29, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.A.; Li, J.; Liu, B.; Hunter, D.; Pyles, M.; Gillette, M.; Dhamdhere, G.R.; Abo, A.; Oro, A.; Helms, J.A. Activating Hair Follicle Stem Cells via R-spondin2 to Stimulate Hair Growth. J. Investig. Dermatol. 2016, 136, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.-I.; Kim, E.-J.; Kim, M.-K.; Jeon, Y.-J.; Kang, S.-M.; Koh, Y.S.; Yoo, E.-S.; Kang, H.-K. The Promoting Effect of Ishige sinicola on Hair Growth. Mar. Drugs 2013, 11, 1783–1799. [Google Scholar] [CrossRef] [Green Version]
- Boisvert, W.A.; Yu, M.; Choi, Y.; Jeong, G.H.; Zhang, Y.-L.; Cho, S.; Choi, C.; Lee, S.; Lee, B.-H. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complement. Altern. Med. 2017, 17, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-Y.; Huang, Y.-C.; Huang, K.-S.; Chan, C.-C.; Chiu, H.-Y.; Tsai, R.-Y.; Chan, J.-Y.; Lin, S.-J. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J. Dermatol. Sci. 2017, 86, 114–122. [Google Scholar] [CrossRef]
- Sennett, R.; Rendl, M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Boil. 2012, 23, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Rendl, M.; Lewis, L.; Fuchs, E. Molecular Dissection of Mesenchymal-Epithelial Interactions in the Hair Follicle. PLoS Boil. 2005, 3, e331. [Google Scholar] [CrossRef]
- Ramos, R.; Guerrero-Juarez, C.F.; Plikus, M.V. Hair follicle signaling networks: A dermal papilla-centric approach. J. Investig. Dermatol. 2013, 133, 2306–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premanand, A.; Rajkumari, B.R. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch. Dermatol. Res. 2018, 310, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.; Tan, J.H.; Bahta, A.W.; Ho, B.S.-Y.; Liu, X.; Lim, T.C.; Sia, Y.Y.; Bigliardi, P.; Heilmann, S.; Wan, A.C.; et al. Differential Expression between Human Dermal Papilla Cells from Balding and Non-Balding Scalps Reveals New Candidate Genes for Androgenetic Alopecia. J. Investig. Dermatol. 2016, 136, 1559–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibino, T.; Nishiyama, T. Role of TGF-β2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef]
- Naruse, T.; Aoki, M.; Fujimoto, N.; Arase, S.; Oura, H.; Ueda, Y.; Ikeda, A. Novel ALK5 inhibitor TP0427736 reduces TGF-β induced growth inhibition in human outer root sheath cells and elongates anagen phase in mouse hair follicles. Pharmacol. Rep. 2017, 69, 485–491. [Google Scholar] [CrossRef]
- Lu, G.-Q.; Wu, Z.-B.; Chu, X.-Y.; Bi, Z.-G.; Fan, W.-X. An investigation of crosstalk between Wnt/β-catenin and transforming growth factor-β signaling in androgenetic alopecia. Medicine 2016, 95, e4297. [Google Scholar] [CrossRef]
- Luo, J.; Chen, M.; Liu, Y.; Xie, H.; Yuan, J.; Zhou, Y.; Ding, J.; Deng, Z.; Li, J. Nature-derived lignan compound VB-1 exerts hair growth-promoting effects by augmenting Wnt/β-catenin signaling in human dermal papilla cells. PeerJ 2018, 6, e4737. [Google Scholar] [CrossRef]
- Bassino, E.; Antoniotti, S.; Gasparri, F.; Munaron, L. Effects of flavonoid derivatives on human microvascular endothelial cells. Nat. Prod. Res. 2016, 30, 2831–2834. [Google Scholar] [CrossRef]
- Madaan, A.; Joshi, V.; Kishore, A.; Verma, R.; Singh, A.T.; Jaggi, M.; Sung, Y.K. In vitro Hair Growth Promoting Effects of Naringenin and Hesperetin on Human Dermal Papilla Cells and Keratinocytes. Am. J. Dermatol. Venereol. 2017, 6, 51–57. [Google Scholar]
- Kamimura, A.; Takahashi, T. Procyanidin B-2, extracted from apples, promotes hair growth: A laboratory study. Br. J. Dermatol. 2002, 146, 41–51. [Google Scholar] [CrossRef]
- Harmon, C.; Nevins, T.; Bollag, W. Protein kinase C inhibits human hair follicle growth and hair fibre production in organ culture. Br. J. Dermatol. 2006, 133, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kamimura, A. Cyclosporin A Promotes Hair Epithelial Cell Proliferation and Modulates Protein Kinase C Expression and Translocation in Hair Epithelial Cells. J. Investig. Dermatol. 2001, 117, 605–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-Y.; Lee, J.; Kapasi, S.; Peterson, S.E.; Gilchrest, B.A.; Gonzalez, S.; Middelkamp-Hup, M.A. Topical Application of a Protein Kinase C Inhibitor Reduces Skin and Hair Pigmentation. J. Investig. Dermatol. 2004, 122, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.; Lee, S.; Kim, S.; Park, D.; Jung, E. Effect of sinapic acid on hair growth promoting in human hair follicle dermal papilla cells via Akt activation. Arch. Dermatol. Res. 2017, 309, 381–388. [Google Scholar] [CrossRef]
- Bejaoui, M.; Villareal, M.O.; Isoda, H. β-catenin-mediated hair growth induction effect of 3,4,5-tri-O-caffeoylquinic acid. Aging 2019, 11, 4216–4237. [Google Scholar] [CrossRef]
- Kim, Y.E.; Choi, H.C.; Lee, I.-C.; Yuk, D.Y.; Lee, H.; Choi, B.Y. 3-Deoxysappanchalcone Promotes Proliferation of Human Hair Follicle Dermal Papilla Cells and Hair Growth in C57BL/6 Mice by Modulating WNT/β-Catenin and STAT Signaling. Biomol. Ther. 2016, 24, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-E.; Choi, H.C.; Nam, G.; Choi, B.Y. Costunolide promotes the proliferation of human hair follicle dermal papilla cells and induces hair growth in C57BL/6 mice. J. Cosmet. Dermatol. 2018, 18, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ryu, S.-W.; Lee, J.; Choi, K.; Kim, S.; Choi, C. Protopanaxatirol type ginsenoside Re promotes cyclic growth of hair follicles via inhibiting transforming growth factor β signaling cascades. Biochem. Biophys. Res. Commun. 2016, 470, 924–929. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Wang, J.; Gao, Y.; Yang, F.; Li, G.; Shi, Q. Ginsenoside Rb1 promotes the growth of mink hair follicle via PI3K/AKT/GSK-3β signaling pathway. Life Sci. 2019, 229, 210–218. [Google Scholar] [CrossRef]
- Kang, J.-I.; Kim, M.-K.; Lee, J.-H.; Jeon, Y.-J.; Hwang, E.K.; Koh, Y.S.; Hyun, J.W.; Kwon, S.-Y.; Yoo, E.-S.; Kang, H.-K. Undariopsis peterseniana Promotes Hair Growth by the Activation of Wnt/β-Catenin and ERK Pathways. Mar. Drugs 2017, 15, 130. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2020, 10, 3. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfur1c acid. Planta Medica 1976, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, O.E.; Olayemi, F.O.; Ning-Hua, T.; Guang-Zhi, Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 10–14. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akula, U.S.; Odhav, B. In vitro 5-Lipoxygenase inhibition of polyphenolic antioxidants from undomesticated plants of South Africa. J. Med. Plant Res. 2008, 2, 207–212. [Google Scholar]
Sample Availability: Samples of the extracts and fractions are available in small quantity from authors upon request. |








| # | Experimental Ion Mass (m/z) | Molecular Formula (Error in ppm) | Tentative Identification | Presence | Comparison Source |
|---|---|---|---|---|---|
| 1 | 153.0195 | C7H6O4 (0.5) | dihydroxybenzoic acid | CEWE, BPLA (adduct) | PR100601 (MassBank) |
| 2 | 173.0455 | C7H10O5 (0.1) | Shikimic acid | CEAE, CEWE | RP017513 (MassBank) |
| 3 | 163.0402 | C9H8O3 (−0.7) | 2-coumaric acid | BEAE, CEAE, FEAE | KO000444 (MassBank) |
| 4 | 301.0354 | C15H10O7 (−1.2) | quercetin | ALL | PB002412 (MassBank) |
| 5 | 359.0771 | C18H16O8 (0.3) | trihydroxy-trimethoxyflavone | BEAE | |
| 6 | 375.1303 | C16H24O10 (−1.6) | loganic acid | FEAE, FEFE | KO001304 (MoNA) VF-NPL−QEHF012680 (MoNA) |
| 7 | 433.1140 | C21H22O10 (0.1) | prunin (naringenin 7-O-glucoside) | CEAE, CEWE | E.I.T. |
| 8 | 447.0933 | C21H20O11 (−0.2) | luteolin-4′-O-glucoside | BEAE | PR100805 (MassBank) |
| 9 | 447.0933 | C21H20O11 (−0.4) | quercitrin (quercetin-3-rhamnoside) | ALL | FIO00587, FIO00586, BS003205 (MoNA) |
| 10 | 447.0933 | C21H20O11 (−1.3) | kaempferol-7-glucoside | BEAE, CEAE, CEWE, CWE | Yamagaki et al., 2014 [34] |
| 11 | 461.1090 | C22H22O11 (−0.2) | scutellarein-7-glucuronide | CEAE, CEWE | BS003571 (MassBank) |
| 12 | 463.1240 | C22H24O11 (1.1) | viscumiside A | BEAE | CCMSLIB0000008176 (MoNA) |
| 13 | 477.0669 | C17H18O13 (0.3) | quercetin-3-O-glucuronide | BEAE | VF-NPL-QTOF009454 (MoNA) RIKENPlaSMA006716 (MoNA) |
| 14 | 489.1414 | C24H26O11 (−2.4) | trimethylisoorientin | CEAE, CEWE, CWE | E.I.T., PR040130 (MoNA) |
| 15 | 511.0554 | C21H20O13S (−1.4) | kaempferol-C6H10O7S | CEWE, CWE | E.I.T. |
| 16 | 527.0500 | C21H20O14S (−1.1) | quercetin-C6H10O7S | CEWE, CWE | E.I.T. |
| 17 | 577.1341 | C30H26O12 (1.9) | procyanidin B2 | CEAE, CEWE, CWE | BS003942 (MassBank) |
| 18 | 579.1360 | C26H28O15 (−0.8) | kaempferol-3-hexoside-pentoside | CEAE, CEWE, CWE | E.I.T. |
| Species | Extracts/Fractions | FRAP Assay | 5-LOX | ||
|---|---|---|---|---|---|
| Trolox Equivalent (μmol.g−1 of Dry Matter) | SD | IC50 (μg mL−1) | Inhibition (% of 5-LOX) | ||
| Calophyllum inophyllum | CWE | 992 | 24 | 55 | 50 |
| CEAE | 1328 | 110 | 88 | 39 | |
| CEAE-F1 | 247 | 19 | 61 | 38 | |
| CEAE-F2 | 415 | 6 | 28 | 50 | |
| CEAE-F3 | 910 | 15 | 20 | 50 | |
| CEAE-F4 | 909 | 28 | 20 | 50 | |
| CEAE-F5 | 2192 | 66 | 21 | 50 | |
| Bidens pilosa | BEAE | 1383 | 103 | 81 | 50 |
| BEAE-F1 | 192 | 12 | 31 | 50 | |
| BEAE-F2 | 536 | 9 | 11 | 8 | |
| BEAE-F3 | 1262 | 20 | 20 | 6 | |
| BEAE-F4 | 3141 | 129 | 57 | 50 | |
| Fagraea berteroana | FEAE | 632 | 49 | 137 | 50 |
| FEAE-F0 | 42 | 2 | - | - | |
| FEAE-F1 | 59 | 3 | 33 | 9 | |
| FEAE-F2 | 175 | 6 | 94 | 19 | |
| FEAE-F3 | 289 | 33 | 74 | 50 | |
| Green tea | 14947 | 868 | |||
| NDGA | 1 | 50 |
| Gene Names | Forward Primer | Reverse Primer |
|---|---|---|
| CALM2 | 5′-GGG-AAC-ATC-TGG-GTT-ATG-CC-3′ | 5′-GAC-TGT-CCA-TAG-TCC-ACG-CA-3′ |
| CCND1 | 5′-AAC-TAC-CTG-GAC-CGC-TTC-CT-3′ | 5′-CCA-CTT-GAG-CTT-GTT-CAC-CA-3′ |
| DKK1 | 5′-TCC-GAG-GAG-AAA-TTG-AGG-AA-3′ | 5′-CCT-GAG-GCA-CAG-TCT-GAT-GA-3′ |
| GAPDH | 5′-CCA-GCA-AGA-GCA-CAA-GAG-GA-3′ | 5′-TGG-TTG-AGC-ACA-GGG-TAC-TT-3′ |
| LEF1 | 5′-GCT-GCC-TAC-ATC-TGA-AAC-ATG-G-3′ | 5′-GGA-TCA-GCG-TCT-CTA-GCA-GT-3′ |
| PPARD | 5′-TTC-CAG-CAG-CTA-CAC-AGA-CC-3′ | 5′-TGA-ACA-CCG-TAG-TGG-AAG-CC-3′ |
| PUM1 | 5′-GGT-GCC-CTT-GTA-GTG-AAT-GC-3′ | 5′-TGT-TGT-TCC-AGC-AAG-ACC-AC-3′ |
| RSPO2 | 5′-CAG-CCT-CAC-ACC-TCT-AGC-AT-3′ | 5′-CTG-CTC-TGC-CCA-GTA-TCT-GT-3′ |
| TGFB1 | 5′-CTG-GCG-ATA-CCT-CAG-CAA-CC-3′ | 5′-CGG-TAG-TGA-ACC-CGT-TGA-TGT-C-3′ |
| WNT5A | 5′-GAG-AGT-GCT-CGC-ATC-CTC-AT-3′ | 5′-GCC-ACA-TCA-GCC-AGG-TTG-TA-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, K.; Ho, R.; Greff, S.; Filaire, E.; Ranouille, E.; Chazaud, C.; Herbette, G.; Butaud, J.-F.; Berthon, J.-Y.; Raharivelomanana, P. Hair Growth Activity of Three Plants of the Polynesian Cosmetopoeia and Their Regulatory Effect on Dermal Papilla Cells. Molecules 2020, 25, 4360. https://doi.org/10.3390/molecules25194360
Hughes K, Ho R, Greff S, Filaire E, Ranouille E, Chazaud C, Herbette G, Butaud J-F, Berthon J-Y, Raharivelomanana P. Hair Growth Activity of Three Plants of the Polynesian Cosmetopoeia and Their Regulatory Effect on Dermal Papilla Cells. Molecules. 2020; 25(19):4360. https://doi.org/10.3390/molecules25194360
Chicago/Turabian StyleHughes, Kristelle, Raimana Ho, Stéphane Greff, Edith Filaire, Edwige Ranouille, Claire Chazaud, Gaëtan Herbette, Jean-François Butaud, Jean-Yves Berthon, and Phila Raharivelomanana. 2020. "Hair Growth Activity of Three Plants of the Polynesian Cosmetopoeia and Their Regulatory Effect on Dermal Papilla Cells" Molecules 25, no. 19: 4360. https://doi.org/10.3390/molecules25194360