Abstract
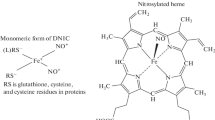
Human erythrocyte hemoglobin (Hb) has two reactive cysteines located on the surface of β-subunits. These cysteines play an important role in the adjustment of Hb functions. It is known that they are involved in the transport of intracellular nitric oxide (NO), redox signaling, and the regulation of dimeric-tetrameric Hb equilibrium. It is shown in this work that the incorporation of Cys-93β as ligands in iron-NO complexes is another way to regulate of SH group reactivity. Such complexes stabilize the SH group as a thiolate anion (R–S–), the reactivity of which is significantly higher than that of the protonated form of thiol (Cys-SH). This is why the thiols included in the complexes show increased activity in relation to electrophilic agents, such as ThioGlo1. Conversely, as part of the complexes, thiols are protected from oxidation by tert-butyl hydroperoxide. The incorporation of SH groups into complexes of iron and NO can be considered a means of thiol protection from irreversible oxidation upon oxidative stress.









Similar content being viewed by others
REFERENCES
Giles, N.M., Watts, A.B., Giles, G.I., Fry, F.H., Littlechild, J.A., and Jacob, C., Chem. Biol., 2003, vol. 10, no. 8, pp. 677–693.
Paulsen, C.E. and Carroll, K.S., ACS Chem. Biol., 2010, vol. 5, no. 1, pp. 47–62.
Paulsen, C.E. and Carroll, K.S., Chem. Rev., 2013, vol. 113, no. 7, pp. 4633–4679.
Go, Y.M., Chandler, J.D., and Jones, D.P., Free Radic. Biol. Med., 2015, vol. 84, pp. 227–245.
Klomsiri, C., Karplus, P.A., and Poole, L.B., Antioxid. Redox Signal., 2010, vol. 14, no. 6, pp. 1065–1077.
Gupta, V. and Carroll, K.S., Biochim. Biophys. Acta, 2013, vol. 1840, no. 2, pp. 847–875.
Novikova, N.N., Kovalchuk, M.V., Yurieva, E.A., Konovalov, O.V., Stepina, N.D., Rogachev, A.V., Yalovega, G.E., Kosmachevskaya, O.V., Topunov, A.F., and Yakunin, S.N., J. Phys. Chem. B, 2019, vol. 123, no. 40, pp. 8370–8377.
Lo, ConteM. and Carroll, K.S., J. Biol. Chem., 2013, vol. 288, no. 37, pp. 26480–26488.
Wible, R.S. and Sutter, T.R., Chem. Res. Toxicol., 2017, vol. 30, no. 3, pp. 729–762.
Kosmachevskaya, O.V., Shumaev, K.B., and Topunov, A.F., Biochemistry (Moscow), 2019, vol. 84, suppl. 1, pp. S206–S224.
Foyer, C.H., Wilson, M.H., and Wright, M.H., Free Radic. Biol. Med., 2018, vol. 122, pp. 137–149.
Vanin, A.F., Nitric Oxide, 2016, vol. 54, pp. 15–29. https://doi.org/10.1016/j.niox.2016.01.006
Vanin, A.F., Dinitrosyl Iron Complexes as a “Working Form” of Nitric Oxide in Living Organisms, Cambridge: Scholars Publishing, 2019.
Reischl, E., Dafre, A.L., Franco, J.L., and Wilhelm Filho, D., Comp. Biochem. Physiol. C Toxicol. Pharmacol., 2007, vol. 146, nos. 1–2, pp. 22–53.
Shumaev, K.B., Gubkin, A.A., Serezhenkov, V.A., Lobysheva, I.I., Kosmachevskaya, O.V., Ruuge, E.K., Lankin, V.Z., Topunov, A.F., and Vanin, A.F., Nitric Oxide, 2008, vol. 18, no. 1, pp. 37–46.
Shumaev, K.B., Kosmachevskaya, O.V., Timoshin, A.A., Vanin, A.F., and Topunov, A.F., Methods Enzymol., 2008, vol. 436, pp. 445–461.
Shumaev, K.B., Petrova, N.E., Zabbarova, I.V., Vanin, A.F., Topunov, A.F., Lankin, V.Z., and Ruuge, E.K., Biochemistry (Moscow), 2004, vol. 69, no. 5, pp. 569–574.
Shumaev, K.B., Dudylina, A.L., Ivanova, M.V., Pugachenko, I.S., and Ruuge, E.K., Biofactors, 2018, vol. 44, no. 3, pp. 237–244.
Shumaev, K.B., Gorudko, I.V., Kosmachevskaya, O.V., Grigoryeva, D.V., Panasenkoe, O.M., Vanin, A.F., Topunov, A.F., Terekhova, M.S., Sokolov, A.V., Cherenkevich, S.N., and Ruuge, E.K., Oxid. Med. Cell. Longev., 2019, vol. 2019, article ID 2798154. https://doi.org/10.1155/2019/2798154
Domanski, A.V., Lapshina, E.A., and Zavodnik, I.B., Biochemistry (Moscow), 2005, vol. 70, no. 7, pp. 761–769.
Shumaev, K.B., Gubkin, A.A., Gubkina, S.A., Gudkov, L.L., Lakomkin, V.L., Topunov, A.F., Vanin, A.F., and Ruuge, E.K., Biophysics (Moscow), 2007, vol. 52, no. 3, pp. 336–339.
Hoff, S., Larsen, F.H., Andersen, M.L., and Lund, M.N., Analyst, 2013, vol. 138, no. 7, pp. 2096–2103.
Davies, K.J. and Delsignore, M.E., J. Biol. Chem., 1987, vol. 262, no. 20, pp. 9908–9913.
Laemmli, U.K., Nature, 1970, vol. 227, no. 5259, pp. 680–685.
Blacken, G.R., Wang, Y., Lopez, J.A., and Fu, X., Blood, 2009, vol. 114, no. 22, pp. 4040–4040.
Riggs, A., J. Biol. Chem., 1961, vol. 236, no. 7, pp. 1948–1954.
Benesch, R.E. and Benesch, R., Biochemistry, 1962, vol. 1, no. 5, pp. 735–738.
Gorbunov, N.V., Osipov, A.N., Day, B.W., Zayas-Rivera, B., Kagan, V.E., and Elsayed, N.M., Biochemistry, 1995, vol. 34, no. 20, pp. 6689–6699.
Gorbunov, N.V., Yalowich, J.C., Gaddam, A., Thampatty, P., Ritov, V.B., Kisin, E.R., Elsayed, N.M., and Kagan, V.E., J. Biol. Chem., 1997, vol. 272, no. 19, pp. 12328–12341.
Reeder, B.J., Grey, M., Silaghi-Dumitrescu, R.-L., Svistunenko, D.A., Bulow, L., Cooper, C.E., and Wilson, M.T., J. Biol. Chem., 2008, vol. 283, no. 45, pp. 30780–30787.
Vlasova, I.I., Molecules, 2018, vol. 23, no. 10. e2561. https://doi.org/10.3390/molecules23102561
Bhattacharjee, S., Deterding, L.J., Jiang, J., Bonini, M.G., Tomer, K.B., Ramirez, D.C., and Mason, R.P., J. Am. Chem. Soc., 2007, vol. 129, no. 44, pp. 13493–13501.
Gunther, M.R., Sturgeon, B.E., and Mason, R.P., Toxicology, 2002, vol. 177, no. 1, pp. 1–9.
Steffek, R.P. and Thomas, M.J., Free Radic. Res. Commun., 1991, vol. 12-13, no. 2, pp. 489–497.
Jia, Y., Buehler, P.W., Boykins, R.A., Venable, R.M., and Alayash, A.I., J. Biol. Chem., 2007, vol. 282, no. 7, pp. 4894–4907.
Laguerre, M., Bily, A., Roller, M., and Birtic, S., Annu. Rev. Food Sci. Technol., 2017, vol. 8, pp. 391–411.
Shumaev, K.B., Gubkin, A.A., Gubkina, S.A., Gudkov, L.L., Sviryaeva, I.V., Timoshin, A.A., Topunov, A.F., Vanin, A.F., and Ruuge, E.K., Biophysics (Moscow), 2006, vol. 51, no. 3, pp. 423–428.
Shumaev, K.B., Gubkina, S.A., Vanin, A.F., Burbaev, D.Sh., Mokh, V.P., Topunov, A.F., and Ruuge, E.K., Biophysics (Moscow), 2013, vol. 58, no. 2, pp. 172–177.
Winterbourn, C.C. and Carrell, R.W., Biochem. J., 1977, vol. 165, no. 1, pp. 141–148.
Pimenova, T., Pereira, C.P., Gehrig, P., Buehler, P.W., Schaer, D.J., and Zenobi, R., J. Proteome Res., 2010, vol. 9, no. 8, pp. 4061–4070.
Stamler, J.S., Singel, D.J., and Piantadosi, C.A., Nat. Med., 2008, vol. 14, no. 10, pp. 1008–1009.
Jensen, F.B., J. Exp. Biol., 2009, vol. 212, no. 21, pp. 3387–3393.
Gaston, B., May, W.J., Sullivan, S., Yemen, S., Marozkina, N.V., Palmer, L.A., Bates, J.N., and Lewis, S.J., J. Appl. Physiol., 2014, vol. 116, no. 10, pp. 1290–1299.
Zhao, Y., Wang, X., Noviana, M., and Hou, M., Acta Biochim. Biophys. Sin., 2018, vol. 50, no. 7, pp. 621–634.
Kosmachevskaya, O.V., Nasybullina, E.I., Blindar, V.N., and Topunov, A.F., Appl. Biochem. Microbiol., 2019, vol. 55, no. 2, pp. 83–98.
O'Neill, J.S. and Reddy, A.B., Nature, 2011, vol. 469, no. 7331, pp. 498–503.
Balagopalakrishna, C., Abugo, O.O., Horsky, J., Manoharan, P.T., Nagababu, E., and Rifkind, J.M., Biochemistry, 1998, vol. 37, no. 38, pp. 13194–13202.
Vitturi, D.A., Sun, C.W., Harper, V.M., Thrash-Williams, B., Cantu-Medellin, N., Chacko, B.K., Peng, N., Dai, Y., Wyss, J.M., Townes, T., and Patel, R.P., Free Radic. Biol. Med., 2013, vol. 55, pp. 119–129.
Jakob, U., Eser, M., and Bardwell, J.C.A., J. Biol. Chem., 2001, vol. 275, no. 49, pp. 38302–38310.
Kaim, W. and Schwederski, B., in Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life, Meyer, G.N.A., Ed., Chichester, UK: Wiley, 1991, pp. 330–350.
ACKNOWLEDGMENTS
The equipment of the Industrial Biotechnologies Center for Collective Use of the Research Center of Biotechnology, Russian Academy of Sciences, was used for the research.
This work was supported by the Russian Foundation for Basic Research (project no. 19-29-12052) and the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by I. Shipounova
Rights and permissions
About this article
Cite this article
Kosmachevskaya, O.V., Nasybullina, E.I., Shumaev, K.B. et al. Effect of Iron–Nitric Oxide Complexes on the Reactivity of Hemoglobin Cysteines. Appl Biochem Microbiol 56, 512–520 (2020). https://doi.org/10.1134/S0003683820050099
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820050099