Abstract
Higher body mass index (BMI) is associated with osteoarthritis (OA) in both weight-bearing and non-weight-bearing joints, suggesting a link between OA and poor metabolic health beyond mechanical loading. This risk may be influenced by systemic factors accompanying BMI. Fluctuations in concentrations of metabolites may mark or even contribute to development of OA. This study explores the association of metabolites with radiographic knee/hip OA prevalence and progression. A 1H-NMR-metabolomics assay was performed on plasma samples of 1564 cases for prevalent OA and 2,125 controls collected from the Rotterdam Study, CHECK, GARP/NORREF and LUMC-arthroplasty cohorts. OA prevalence and 5 to 10 year progression was assessed by means of Kellgren-Lawrence (KL) score and the OARSI-atlas. End-stage knee/hip OA (TJA) was defined as indication for arthroplasty surgery. Controls did not have OA at baseline or follow-up. Principal component analysis of 227 metabolites demonstrated 23 factors, of which 19 remained interpretable after quality-control. Associations of factor scores with OA definitions were investigated with logistic regression. Fatty acids chain length (FALen), which was included in two factors which associated with TJA, was individually associated with both overall OA as well as TJA. Increased Fatty Acid chain Length is associated with OA.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is very prevalent, age-related, progressive degenerative disease of articular joints and one of the leading causes of disability and pain worldwide1. Due to ageing, increased longevity, and increasing obesity of the population, the OA incidence is expected to rise even further2,3,4. Epidemiological studies have shown that unfavourable metabolic parameters, such as high body mass index (BMI), waist-hip ratio and proportion of fat mass associated with OA onset and its severity5,6,7,8,9,10,11. Conversely, weight loss has been shown to significantly relief pain while increasing physical function of people with knee OA12,13,14,15. Notably, these associations have been found for both the weight-bearing joint of the knee and non-weight bearing joints of the hand, suggesting a connection between OA and obesity beyond increased loading16,17,18. In line with this, OA has been shown to associate with classical markers of poor metabolic health such as increased circulating levels of low density lipoprotein (LDL) and cholesterol19,20. However, this connection remains controversial for the hip joint21.
Recently, studies investigated metabolic profiles to identify biomarkers of poor metabolic health in association with OA and its progression. Few small studies were performed into circulating serum metabolomics biomarkers, identifying that ratios of amino acids such as valine and xleucine to histidine and alanine, arginine, aspartate, glutamate and proline are associated with OA22,23,24,25. More studies have been performed using synovial fluid which have confirmed the some of the aforementioned amino acids, as well as identifying altered energy-, collagen-, fatty acid-, glycerolipid-, oxidative stress and nitric oxide-metabolisms26,27,28 suggesting an altered metabolic state of the synovial fluid in OA. However, these hallmarks of the metabolic syndrome (MS) should also be present in serum as MS affects not only the synovium but also the whole body. In addition, consensus on biomarkers is lacking and the sample size of these studies was generally small. Warranting the need to perform large-scale studies to elucidate the link between metabolites and OA.
Therefore, in the current study, we set out to conduct the first large scale metabolomics assessment including metabolites ranging from amino acids to lipids to fatty acids in over four thousand cases and controls. We aim to identify metabolic signatures of serum by including 2,125 controls and 2,372 OA cases among Dutch cohorts participating in the Biobanking and BioMolecular resources Research Infrastructure consortium (BBMRI metabolomics consortium)29.
Results
Levels of 231 serum metabolic traits (Supplementary Table 2) were obtained on the NMR (Nightingale) platform. Of the 231 metabolites, 4 [conjugated linoleic acid (CLA), the ratio of CLA to total fatty acids (CLAFA), diglycerids (DAG) and ratio DAG to triglycerids (DAG/TG)] had more than 5% of the measurements below the detection limit and were excluded from analysis. Analyses of the 227 remaining metabolites was performed in 2,125 controls and 1,556 OA cases from 4 independent studies participating in the Biobanking and BioMolecular resources Research Infrastructure consortium (BBMRI metabolomics consortium, Supplementary Table 1). Baseline characteristics of cases and controls are shown in Table 1.
Principal component analysis (PCA)
To reduce data dimensions of the correlated metabolites, PCA analyses was performed. In a PCA analysis the underlying the structure of correlation between individual metabolites is taken into account and merged into independent factors. As shown in Supplementary Table 3, 23 factors with an eigenvalue of ≥ 1, accounting for 91,4% of the total variance were identified. Indicating that in the 227 assessed metabolites, 23 independent correlation structures are present. Prior to the analyses for OA status, possible confounding effects of cohorts were investigated by analysing the principal components as function of controls of each cohort while adjusting for age, sex, BMI and fasting status. Hereto four factors (factor-2, factor-10, factor-13 and factor-16), accounting for 28.06% of the variance were removed from the analyses.
Cross-sectional analyses of prevalent overall OA
After adjusting for age, gender BMI and multiple testing, we identified five factors (factor-4, factor-11, factor-15 factor-19, and factor-20) that were significantly associated to overall OA as defined by a KL score of ≥ 2 or having a total joint arthroplasty in hip and/or knee joints (Table 2).
Of these, most significantly associated to overall OA were factor-4 with OR 1.25 95% CI 1.15–1.36, P < 0.001 and factor 19 OR 0.75, 95% CI 0.69–0.82, P < 0.001. In order to assess the type of biology that each factor represents, we looked at the individual correlated metabolites for each factor. Based on the metabolites that, among others, load on these factors (Supplementary Table 2) we conclude that factor-4 represents mainly the medium and large triglycerides to total lipids ratio and factor 19 particularly the amino acids glutamine and histidine). The metabolites that represent factor-11 and factor-20 are different types of fatty acid ratios and 3-hydroxybutyrate and citrate, respectively. Different cholesterols represent factor-15. Associations separate for hip and knee OA were comparable (Supplementary Table 4).
Cross-sectional analyses total joint arthroplasty
To investigate associations among the part of the cases representing patients with a total joint arthroplasty surgery, and therefore symptomatic end-stage disease, we performed similar cross-sectional analyses with total joint arthroplasty. Even though these analyses had less power than the cross-sectional analyses for overall OA due to a smaller sample size, the effect sizes were more pronounced and the levels of significance were comparable to the overall OA analyses. After adjusting for age, gender, BMI and multiple testing, we identified eight factors (factor-1, factor-4, factor-6, factor-11, factor-17, factor-19, factor-22, and factor-23) that were significantly associated to patients with joint arthroplasty of knee and/or hip (Table 2).
Notably, factor-1, factor-6, factor-17, factor-22, and factor-23 did not show association to overall OA and may thus be specifically associated with arthroplasties. These associations were slightly more prominent for arthroplasty in the knee as compared to hip arthroplasty (Supplementary Table 5). Based upon the metabolites loading on these factors we conclude that factor-1 is mainly representative of the LDL/VLDL metabolism, factor -6 represents cholesterol(esters), factor-17 and factor-23 represent different aspects of the energy cycle and factor-22 represents fatty acid make-up.
To further assess the confounding effect of fasting-status, we performed additional analyses in- and excluding adjustment for fasting. As shown in Supplementary Table 6, the association of the factors with OA remained consistent with and without an adjustment for fasting. Also, upon splitting the cohort in those with BMI < 30 and BMI ≥ 30, most factors showed similar odds ratio’s between the two groups. Notably, the effect of factor 4 and 17 was slightly larger in the more obese group while factor 19 was stronger in those with BMI < 30. Factor 22 was the most robust with comparable Odds Ratio’s between the two groups (Supplementary Table 7).
Cross-sectional analyses of individual metabolites
Factors 1, 4, 6, 11, 17, 19, 22 and 23, which were associated with arthroplasty, were selected for further in-depth analysis. To investigate whether any of the individual metabolites within the identified factors drive the associations, we assessed the association with the individual metabolites significantly represented in these factors (loading on the factor > 0.4 or < − 0.4). As shown in Supplementary Table 8, the majority of the individual metabolites in the factors were associated to a similar extent (OR and P-value) to arthroplasty and as such reflect high correlation between the individual metabolite features within a factor. A notable exception, however, is FALen (Fatty Acid chain Length) represented in factor-1 and factor-22. The association with the individual FALen metabolite stands out as it appears to give the strongest and most specific association with TJA (OR 1.83, 95% CI 1.64–2.05, P < 0.001, see also Table 3. In order to assess any differences in the association of FALen and joint site, we have assessed the association of FALen with different joint sites and phenotypes. As shown in Table 3, FALen was significantly associated with all included phenotypes, with the strongest association found for knee arthroplasty. Additionally, as shown in Supplementary Table 7, factor 22 driven by FALen is stable across the different BMI strata. As statins might affect the concentrations of metabolites, we performed sensitivity analyses to assess their influence on our outcomes. The use of statins was known for all included studies except CHECK. Sensitivity analysis revealed only minor changes in the effect sizes. Similarly, upon in- and excluding an adjustment for statins in the analyses, the Odds Ratio’s changed marginally.
Progression of OA
As FALen showed strong associations with TJA as well as with overall OA (OR 1.26, 95% CI 1.16–1.36, P < 0.001), we next explored its association with the progression of OA. Progression data was available for N = 2009 (N = 721 non-progressors and N = 305 progressors in the Rotterdam Study and N = 522 non-progressors and N = 130 progressors in CHECK) (Materials and Methods). As shown in (Supplementary Fig. 1) and despite the high and consistent cross sectional association the individual metabolite FALen was not associated to OA progression.
Discussion
Serum metabolomic assays of hip and knee OA cases and controls were assessed by means of the Nightingale 1H-NMR platform, resulting in 227 different metabolite measurements. PCA-analysis identified 23 factors explaining 71.9% of variance, of which 19 were eligible for further regression analyses. Eight factors (factor-1, factor-4, factor-6, factor-11, factor-17, factor-19, factor-22 and factor-23) were associated with total joint arthroplasty independent of age, sex and BMI. Assessing the association of individual metabolites of these factors with TJA revealed a highly significant association between Fatty Acid Chain Length (FALen) and Any OA or TJA (P < 0.001). Upon splitting the phenotypes by joint site (hip or knee) consistent, significant positive association was found for FALen and all four additional phenotypes. As such, this study places a composite score of fatty acid length at the heart of the link of osteoarthritis and metabolites. This study showed that FALen has a strong association with end stage OA as represented by overall radiographic OA (OR 1.26) but particularly to joint arthroplasty (OR 1.83).
Recently, the comprehensive review by Harasymowicz et al. described the many ways how fatty acids influence not only the cartilage, but also bone and synovial fluids in the joint30. Specifically, they describe that longer chain saturated fatty acids have a negative impact on the homeostasis of cartilage and chondrocyte metabolism. In addition, long-chain SFA inhibits osteoclast-genesis, whereas shorter-chain SFA enhance osteoclast-genesis30,31. Another recent study has shown that longer-chain dietary fatty acids in rats induce both metabolic syndrome and changes in the knee joint resembling OA32, all confirming our finding that longer chains of the fatty acid are associated with end stage, symptomatic OA. Fatty acids are known to play a role in a broad range of cardiovascular diseases as well as to the immune system, which might hint that there is a more generic pathway underlying the association of OA and fatty acid make up33,34. This finding was supported by the study of Senol et al. in OA patients which demonstrated that there are differences in the metabolic pathways of fatty acid biosynthesis pathways between OA patients and controls35. Given these studies, we advocate that the length of the Fatty Acid Chain is underlying the risk of OA susceptibility by directly affecting chondrocyte energy metabolism. Indeed, pathways which are activated during high concentrations of fatty acid in blood such as mTOR and AMPK are found to be associated with knee OA in mouse. Leading to increased apoptosis in mouse knee and aging of the murine cartilage36. Reduced AMPK is associated with a loss of mitochondrial function, which in turn leads to ageing of the cartilage37. Again, this indicates a more generic pathway underlying the association of fatty acids with OA38.
We did not demonstrate any association of FALen with OA progression. This may be explained by the fact that OA progression was defined by KL-scores alone, as such differ as the symptomatic end stage OA of patients undergoing arthroplasty. For that matter, in Supplementary Fig. 1 it is suggested that the effect for FALen and OA-progression is larger in the CHECK cohort as compared to that in the Rotterdam study as inclusion criteria of CHECK patients were based on symptoms and in the Rotterdam Study based on radiographic OA alone.
FALen was present in both Factor-1 and Factor-22, the first explaining a large part of the variance in data (31.25%), whereas factor-22 explains 0.49%. The fact that FALen is present in both and that both are significantly associated with TJA, shows that it is worthwhile to explore also small factors. Notably, two other metabolites that were represented in factor-22, hence showing an underlying correlation structure to FALen, were Degree of Unsaturation (UnsatDeg) and ratio of saturated fatty acids (SFA FA). Nonetheless, the absence of association of these metabolites to TJA (Supplementary Table 8) confirms that the found association of Factor-22 to TJA (OR 1.50, 1.33–1.70) is almost totally attributional to fatty acid chain length (FALen; OR 1.83, 1.64–2.05) and not to fatty acid saturation (UnsatDeg OR 1.12, 95% CI 0.99–1.28 and SFA FA OR 0.95 95% CI 0.84–1.07).
The fact that FALen is also present in Factor-1, alongside many other metabolites in the LDL/VLDL range, shows that besides its own specific interaction with OA, FALen plays a role through lipid-transportation, as is reflected by Factor-130. This double presence shows the highly correlated interplay of metabolites and reflects the fact that the length of a fatty acid chain length alters the characteristics of the fatty acid, influencing the metabolism. Although studies have shown that the link between OA and obesity is more often present in knee OA, we found in this study that FALen is associated to both knee and hip OA, with the strongest association found for knee replacement surgery. This may indicate a more general link between OA and fatty acid length, beyond obesity and knee OA which is also supported by the stable associations of factor 22 (driven by FALen) across the BMI strata (Supplementary Table 7).
In this study, we choose to differentiate between patients who underwent total hip or knee arthroplasty from patients with radiographic signs of OA. This because arthroplasty-patients are essentially in a different (end)stage of the disease, and an additional incentive to undergo joint replacement surgery has taken place. In contrast, patients with radiological OA represent a range of patients with symptoms who may not (yet) be severe enough for an indication for arthroplasty. The fact that we observed stronger associations with arthroplasty patients, in spite of lower power, supports the view that deviation of metabolic profiles gradually accompanies aspects of the OA process towards a joint replacement surgery. However, disentangling the relationship between FALen, OA symptoms, and joint replacement requires additional in depth investigation preferably in studies in which symptoms, radiographic OA and the incentive towards a joint replacement surgery is accurately documented.
A strength of our study was the relatively large sample size as compared to reported studies. The combination of different studies to reach more power also meant that we incorporated some studies with only cases or controls, raising the chance of cohort effects. However, we did assess the presence of cohort effects within the control group, where no differences should be present between the cohorts as all samples are OA-free. Only factors free of cohort effects were included in our analyses. We adjusted our analyses for baseline-BMI; however, we cannot exclude bias in our findings due to the effect of weight loss or gain during follow-up. Our study included both fasted and nonfasted samples while metabolites may be sensitive to fasting status, therefore we assessed the influence of including a correction or fasting status to assess dietary influences. Despite the fact that food intake influences metabolite patterns the association with OA remained untouched39. We demonstrated only marginal changes, i.e. effect sizes were very similar.
The current paper is to our knowledge the first large scale hypothesis free approach in search for metabolites that associate to OA in a cross-sectional design. Future research should particularly focus on replication of the found results and, if this succeeds, further elucidate the mechanisms behind the association of fatty acid chain length and OA.
In conclusion, the current study identified a number of metabolic factors associated with OA, independent of BMI. This indicates that there is an altered metabolic state in patients with OA as compared to controls without OA. This is another token that OA should be seen as a component of poor metabolic health and that recording systemic factors of health status in older people may represent an informative marker of OA risk.
Methods
Study populations
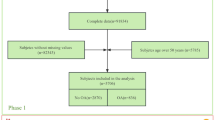
Metabolic signatures were assessed in N = 2,125 controls and N = 1,556 cases from 4 Dutch cohorts being Cohort Hip & Cohort Knee (CHECK, N = 864), Genetics, Arthrosis and Progression (GARP-study, N = 216) with its NORmal REFerence Controls (NORREF, N = 34), Leiden University Medical Centre (LUMC) Arthroplasty studies (LUMC-cohorts N = 455) and the Rotterdam study (N = 2,112) that participated in the Biobanking and BioMolecular resources Research Infrastructure consortium (BBMRI metabolomics consortium)29. Distribution of cases and controls across these studies is depicted in Supplementary Table 1. Informed consent was obtained from all included participants. The experiment protocol was followed according to the Declaration of Helsinki and good clinical practice. In addition, this study was assessed by the steering committees of each included study and the Medical Ethics Committees of all participating centres approved the study [Albert Schweitzer Hospital (Dordrecht), Atrium Medisch Centrum (Heerlen), Canisius-Wilhelmina Ziekenhuis (Nijmegen), Erasmus Medical Center (Rotterdam), Groene Hart Hospital (Gouda), Isala Klinieken (Zwolle), Kennemer Gasthuis (Haarlem), Leyenburg ziekenhuis (The Hague) Leids Universitair Medisch Centrum (Leiden), Maastricht University Medical Center (Maastricht), Martini Ziekenhuis (Groningen), Medisch Centrum Alkmaar (Alkmaar), Medisch Centrum Haaglanden (The Hague), Medisch Spectrum Twente (Enschede), Reade (Amsterdam), Reinier de Graaf Groep (Delft), Rijnland ziekenhuis (Leiderdorp), Slotervaart Hospital (Amsterdam), St. Antonius ziekenhuis (Nieuwegein), St. Joseph Ziekenhuis (Veldhoven), St. Maarten's Gasthuis (Venlo) Tweesteden Ziekenhuis (Tilburg), Universitair Medisch Centrum Utrecht (Utrecht), Ziekenhuis Rijnstate (Arnhem)].
CHECK is a prospective, 10-year follow-up, observational cohort study of 1,002 people aged between 45 and 65 years at the time of inclusion, with pain in their knee(s) and/or hip(s), who had never or not longer than 6 months ago visited a general physician for these complaints40. Blood samples were taken non-fasted. Hip and knee radiographs were scored pairwise according to the Kellgren & Lawrence (KL) scoring system41. When scored pairwise, these people did not have obvious radiographic knee or hip OA at baseline (i.e. KL = 0 or 1). As such, these persons were considered controls for the cross-sectional analyses on OA prevalence at baseline. For the current study we included 864 persons from the CHECK Cohort.
GARP/NORREF study: The GARP cohort (N = 216) consists of patients with advanced radiographic OA at two or more joint sites of hand, spine, knee or hip. Matched to the GARP study, a normal reference control group (NORREF) was collected using the same protocol and included in this study as controls (N = 34)42,43,44. In the current study baseline non-fasting plasma samples were included.
LUMC cohorts: The LUMC arthroplasty studies (N = 455) consist of participants of the “Research Arthritis and Articular Cartilage” (RAAK), “Transfusion induced complications = Transfusion associated complications?” (Tactics) (NTR309) and “Transfusie op Maat” (TOMaat) (NTR303) studies45,46. These cross-sectional studies included OA patients who received THA or TKA. Blood samples were collected during surgery while patients were fasted.
Rotterdam Study: The Rotterdam Study (RS) is a large prospective population-based cohort study of men and women aged 55 years and older in the municipality of Rotterdam, the Netherlands. The study design and rationale are described elsewhere in detail47. In summary, the objective of the study is to investigate determinants, incidence and progression of chronic disabling diseases in the elderly. Baseline measurements were obtained through a home interview and visits to the research centre for physical examinations and imaging and laboratory assessments, blood samples were taken while patient was fasted. The present study includes 2,112 baseline participants from RS-I (Ergo 4). The study included both individuals with and without OA with a mean follow-up time of 6.5 year. Blood was sampled nonfasted. The study included both individuals with and without OA at baseline with mean follow-up time of 6.5 years.
Definitions of OA
Prevalent radiographic OA was (N = 1556) defined as either having a KL score of ≥ 2 in hip and/or knee at baseline or having THA or TKA for primary OA41. THA/TKA patients (N = 640) were also assessed separately. Controls (N = 2,125) had no radiographic hip and knee OA (KL < 2) and were selected from the Rotterdam Study, CHECK and NORREF as described above. Controls were selected from CHECK and the Rotterdam Study and had no radiographic signs of OA in knee or hip. Data on radiographic OA progression were available for CHECK (10 year FU), and the Rotterdam Study (6.5 year FU). Progression was defined as any increase in KL score, resulting in a KL score of ≥ 2 at follow-up. Thus, both incidence (KL score of 0 or 1 at baseline and ≥ 2 at follow-up) and progression (increase at KL score with a baseline of ≥ 2) were defined as progression in our analyses.
Metabolite measurement
EDTA plasma samples were shipped to Nightingale to perform standardized metabolomics analyses on a high throughput platform (Nightingale Ltd, Helsinki, Finland). The 1H-NMR technique used by Nightingale provides simultaneous quantification of routine lipids, lipoprotein subclass profiling with lipid concentration units, resulting in 231 measurements. Details of the techniques have been described before48,49.
Statistical analyses
Metabolites with more than 5% below detection limit were removed from analysis. Principal component analysis (PCA) was applied to reduce the data dimension of correlated metabolites. PCA groups variables (metabolites) into factors in order to explain variance present in the data. Factors with an eigenvalue above 1 after Varimax rotation with Kaiser Normalization were included in analyses. A metabolite was said to load on a given factor if its factor loading was > 0.4 or < − 0.4. For each subject, a score was computed for the measures loaded on the factor, representing the linear relationship (Pearson correlation under Varimax rotation) between a factor and variable.
The remaining factors were included in logistic regression analyses to assess their association with OA, adjusting for age, sex and BMI and multiple testing. As it is hard to interpret the overall meaning of a factor, the individual metabolites of factors with significant associations to both OA and TJA were included in individual logistic regression analyses after LN-transformation and Z-standardization. All P-values were adjusted according to Bonferroni.
Since follow-up was performed at different time points, the progression analyses were done by means of meta-analysis. The summary statistics (regression coefficients and standard errors) of CHECK were combined with the summary statistics of the Rotterdam Study in a random effects meta-analysis. To assess the modifying role of fasting, analyses were also performed without an adjustment. Analyses were performed by means of IBM SPSS Statistics version 24 and the “meta-package” of R.
References
Litwic, A., Edwards, M. H., Dennison, E. M. & Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 105, 185–199 (2013).
Kurtz, S., Ong, K., Lau, E., Mowat, F. & Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint. Surg. Am. 89, 780–785 (2007).
Lawrence, R. C. et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 58, 26–35 (2008).
Hruby, A. & Hu, F. B. The epidemiology of obesity: a big picture. Pharmacoeconomics 33, 673–689 (2015).
Visser, A. W. et al. The role of fat mass and skeletal muscle mass in knee osteoarthritis is different for men and women: the NEO study. Osteoarthr. Cartil. 22, 197–202 (2014).
Sowers, M. F. et al. BMI vs. body composition and radiographically defined osteoarthritis of the knee in women: a 4-year follow-up study. Osteoarthr. Cartil. 16, 367–372 (2008).
Zhou, Z.-Y., Liu, Y.-K., Chen, H.-L. & Liu, F. Body mass index and knee osteoarthritis risk: a dose-response meta-analysis. Obesity 22, 2180–2185 (2014).
Davis, M. A., Ettinger, W. H., Neuhaus, J. M., Cho, S. A. & Hauck, W. W. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am. J. Epidemiol. 130, 278–288 (1989).
Wang, Y. et al. Relationship between body adiposity measures and risk of primary knee and hip replacement for osteoarthritis: a prospective cohort study. Arthritis Res. Ther. 11, R31 (2009).
Lohmander, L. S., de Verdier, M. G., Rollof, J., Nilsson, P. M. & Engström, G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann. Rheum. Dis. 68, 490–496 (2009).
Nieves-Plaza, M., Castro-Santana, L. E., Font, Y. M., Mayor, A. M. & Vilá, L. M. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J. Clin. Rheumatol. 19, 1–6 (2013).
Muthuri, S. G., Hui, M., Doherty, M. & Zhang, W. What if we prevent obesity? Risk reduction in knee osteoarthritis estimated through a meta-analysis of observational studies. Arthritis Care Res. 63, 982–990 (2011).
Felson, D. T., Zhang, Y., Anthony, J. M., Naimark, A. & Anderson, J. J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham study. Ann. Intern. Med. 116, 535–539 (1992).
Messier, S. P. et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 310, 1263–1273 (2013).
Felson, D. T. & Chaisson, C. E. Understanding the relationship between body weight and osteoarthritis. Baillieres Clin. Rheumatol. 11, 671–681 (1997).
Yusuf, E. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann. Rheum. Dis. 69, 761–765 (2010).
Dahaghin, S., Bierma-Zeinstra, S. M. A., Koes, B. W., Hazes, J. M. W. & Pols, H. A. P. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam study. Ann. Rheum. Dis. 66, 916–920 (2007).
Visser, A. W. et al. Adiposity and hand osteoarthritis: the Netherlands epidemiology of obesity study. Arthritis Res. Ther. 16, R19 (2014).
Curtis, J. R., John, A. & Baser, O. Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti-tumor necrosis factor therapy. Arthritis Care Res. 64, 1282–1291 (2012).
Stürmer, T. et al. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J. Rheumatol. 25, 1827–1832 (1998).
Grotle, M., Hagen, K. B., Natvig, B., Dahl, F. A. & Kvien, T. K. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 9, 1 (2008).
Zhai, G. et al. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann. Rheum. Dis. 69, 1227–1231 (2010).
Zhang, W. et al. Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthr. Cartil. 24, 827–834 (2016).
Chen, R. et al. Perturbations in amino acids and metabolic pathways in osteoarthritis patients determined by targeted metabolomics analysis. J. Chromatogr. B 1085, 54–62 (2018).
Hu, T. et al. An evolutionary learning and network approach to identifying key metabolites for osteoarthritis. PLoS Comput. Biol. 14, e1005986 (2018).
Kim, S. et al. Metabolite profiles of synovial fluid change with the radiographic severity of knee osteoarthritis. Joint Bone Spine 84, 605–610 (2017).
Carlson, A. K. et al. Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochem. Biophys. Res. Commun. 499, 182–188 (2018).
Zheng, K. et al. Global and targeted metabolomics of synovial fluid discovers special osteoarthritis metabolites. J. Orthop. Res. 35, 1973–1981 (2017).
Mayrhofer, M. T., Holub, P., Wutte, A. & Litton, J.-E. BBMRI-ERIC: the novel gateway to biobanks From humans to humans. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 59, 379–384 (2016).
Harasymowicz, N. S., Dicks, A., Wu, C.-L. & Guilak, F. Physiologic and pathologic effects of dietary free fatty acids on cells of the joint. Ann. N. Y. Acad. Sci. 1440, 36–53 (2019).
Cornish, J. et al. Modulation of osteoclastogenesis by fatty acids. Endocrinology 149, 5688–5695 (2008).
Sekar, S. et al. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 7, 46457 (2017).
Baum, S. J. et al. Fatty acids in cardiovascular health and disease: a comprehensive update. J. Clin. Lipidol. 6, 216–234 (2012).
de Jong, A. J., Kloppenburg, M., Toes, R. E. M. & Ioan-Facsinay, A. Fatty acids, lipid mediators, and T-cell function. Front. Immunol. 5, 483 (2014).
Senol, O., Gundogdu, G., Gundogdu, K. & Miloglu, F. D. Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clin. Rheumatol. https://doi.org/10.1007/s10067-019-04428-1 (2019).
Caramés, B., Taniguchi, N., Otsuki, S., Blanco, F. J. & Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62, 791–801 (2010).
Petursson, F. et al. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res. Ther. 15, R77 (2013).
Berenbaum, F., Griffin, T. M. & Liu-Bryan, R. Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol. 69, 9–21 (2017).
Schutte, B. A. M. et al. The effect of standardized food intake on the association between BMI and 1H-NMR metabolites. Sci. Rep. 6, 1 (2016).
Wesseling, J. et al. CHECK (cohort hip and cohort knee): similarities and differences with the Osteoarthritis Initiative. Ann. Rheum. Dis. 68, 1413–1419 (2009).
Kellgren, J. H. & Lawrence, J. S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16, 494–502 (1957).
Riyazi, N. et al. Evidence for familial aggregation of hand, hip, and spine but not knee osteoarthritis in siblings with multiple joint involvement: the GARP study. Ann. Rheum. Dis. 64, 438–443 (2005).
Meulenbelt, I. et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthr. Cartil. 15, 379–385 (2007).
Bijsterbosch, J. et al. Clustering of hand osteoarthritis progression and its relationship to progression of osteoarthritis at the knee. Ann. Rheum. Dis. 73, 567–572 (2014).
Ramos, Y. F. M. et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS ONE 9, e103056 (2014).
So-Osman, C. et al. Patient blood management in elective total hip- and knee-replacement surgery (part 2): a randomized controlled trial on blood salvage as transfusion alternative using a restrictive transfusion policy in patients with a preoperative hemoglobin above 13 g/dl. Anesthesiology 120, 852–860 (2014).
Hofman, A. et al. The Rotterdam Study: 2016 objectives and design update. Eur. J. Epidemiol. 30, 661–708 (2015).
Soininen, P., Kangas, A. J., Würtz, P., Suna, T. & Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 8, 192–206 (2015).
Ala-Korpela, M. Potential role of body fluid 1H NMR metabonomics as a prognostic and diagnostic tool. Expert Rev. Mol. Diagn. 7, 761–773 (2007).
Funding
This work was performed within the framework of the BBMRI Metabolomics Consortium funded by BBMRI-NL, a research infrastructure financed by the Dutch government (NWO, Grant Nr. 184.021.007 and 184033111). We would like all participants of the included studies. CHECK: CHECK was funded by the Dutch Arthritis Association on the lead of a steering committee comprising 16 members with expertise in different fields of OA, chaired by Professor JWJ Bijlsma and coordinated by J Wesseling. Involved are: Erasmus Medical Center Rotterdam; Kennemer Gasthuis Haarlem; Leiden University Medical Center; Maastricht University Medical Center; Martini Hospital Groningen/Allied Health Care Center for Rheumatology and Rehabilitation Groningen; Medical Spectrum Twente Enschede; Reade Amsterdam, St. Maartenskliniek Nijmegen; University Medical Center Utrecht and Wilhelmina Hospital Assen. GARP: Leiden University Medical Centre, Pfizer Groton, Connecticut, USA and the Dutch Arthritis Society have supported the GARP study. Furthermore, the research leading to these results has received funding from the Biobanking and BioMolecular resources Research Infrastructure the Netherlands (BBMRI-NL) (Complementation Project CP2013-84), the Dutch Scientific Research council NWO/ZonMW VICI scheme (nr. 91816631/528) and the Dutch Arthritis Society. We are indebted to drs. N. Riyazi, J. Bijsterbosch, H.M. Kroon and I. Watt for collection of data. The LUMC arthroplasty studies: This was a combination of TACTICS, TOMAAT and RAAK cohorts. TACTICS was funded by The Dutch Board of Health Care Insurances (College voor Zorgverzekeringen; OG99/023) and Sanquin Blood Bank. Involved were Prof. dr R.G.H.H. Nelissen, MD, Prof. dr A. Brand, MD, Leiden University Medical Centre; R.L. te Slaa MD, Reinier de Graaf Gasthuis, Delft; Dr R.G. Poll MD, Slotervaart ziekenhuis, Amsterdam; Dr K.M. Veenstra Franciscus ziekenhuis, Rotterdam and Prof. dr D. van Rhenen Sanquin Blood Bank, Rotterdam. Funding for the TOMAAT-study was received from ZonMW (06-601) and Sanquin Blood Supply (03-002), the Netherlands. Clinical Trial Number: ISRCTN96327523 (controlled-trials.com) and NTR 303 (Dutch Trial Register). The Leiden University Medical Centre have and are supporting the RAAK-study. Furthermore, the research leading to these results has received funding from the Dutch Arthritis Society (DAA_10_1-402), Biobanking and BioMolecular resources Research Infrastructure The Netherlands (BBMRI-NL) complementation project CP2013-83 and Dutch Scientific Research council NWO /ZonMW VICI scheme (Nr. 91816631/528). Rotterdam Study: The Rotterdam Study is supported by the Netherlands Organization of Scientific Research NWO Investments (Nr. 175.010.2005.011, 911-03-012), the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO) (Project Nr. 050-060-810) and the Erasmus Medical Center and Erasmus University, Rotterdam. This study is funded by the Dutch Arthritis foundation (Project Nr. 13-1-201).
Author information
Authors and Affiliations
Contributions
Conception/design: C.M.v.D., P.E.S., J.B.J.v.M., I.M. Data acquisition: N.B., J.G.v.B., J.A.v.H., W.E.v.S., C.S.-O., A.U., M.K., R.G.H.H.N., C.M.v.D., P.E.S., J.B.J.v.M., I.M. Data analysis: J.M.T.A.M., F.S.H., N.B., W.d.H., P.E.S., J.B.J.v.M, I.M. Data interpretation: J.M.T.A.M., F.S.H., P.E.S., J.B.J.v.M., I.M. Drafted the work: J.M.T.A.M., F.S.H., J.B.J.v.M., I.M. Substantially revised: J.M.T.A.M., F.S.H., W.E.v.S., M.K., P.E.S., J.B.J.v.M., I.M. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meessen, J.M.T.A., Saberi-Hosnijeh, F., Bomer, N. et al. Serum fatty acid chain length associates with prevalent symptomatic end-stage osteoarthritis, independent of BMI. Sci Rep 10, 15459 (2020). https://doi.org/10.1038/s41598-020-71811-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71811-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.