Abstract
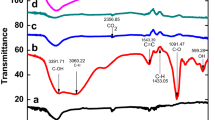
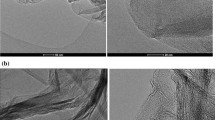
Graphene oxide–supported β-cyclodextrin was prepared with graphene oxide and β-cyclodextrin as raw materials and epichlorohydrin as crosslinking agent, respectively. It was characterized by the methods of Raman spectroscopy, FT-IR, and SEM. The graphene oxide–supported β-cyclodextrin showed excellent adsorption performance for p-nitrophenol, and the absorption equilibrium can be achieved within 2 h. The adsorptive capacity is 117.28 mg/g at adsorption temperature of 313 K and pH at 8.0. Adsorption isotherms showed that the adsorption capacity increases with the increases of temperature and adsorption process could be better fitted by Langmuir isotherm (R2 > 0.995). Thermodynamic functions (ΔG, ΔH, and ΔS) investigation showed that the adsorption is spontaneous, endothermic, and random. The adsorption kinetics of p-nitrophenol over graphene oxide–supported β-cyclodextrin is conformed to a pseudo-second-order process. This study has suggested that the graphene oxide–supported β-cyclodextrin could play an efficient and beneficial source of the adsorbent for the purpose of eliminating p-nitrophenol from aqueous solution.









Similar content being viewed by others
References
Ahmed, M. J., & Theydan, S. K. (2015). Adsorptive removal of p-nitrophenol on microporous activated carbon by FeCl3 activation: equilibrium and kinetics studies. Desalin Water Treat, 55, 522–531.
Badruddoza, A. Z. M., Hazel, G. S. S., Hidajat, K., & Uddin, M. S. (2010). Synthesis of carboxymethyl-β-cyclodextrin conjugated magnetic nano-adsorbent for removal of methylene blue. Colloid Surf A, 367, 85–95.
Barker, G., Calzada, J., Ouyang, Z., Domagalski, N., Herzer, S., & Rieble, S. J. (2016). A systematic approach to improve data quality in high-throughput batch adsorption experiments. Eng Life Sci, 16, 124–132.
Cao, Y., Fatemi, V., Demir, A., Fang, S., Tomarken, S. L., Luo, J. Y., Sanchez-Yamagishi, J. D., Watanabe, K., Taniguchi, T., Kaxiras, E., Ashoori, R. C., & Jarillo-Herrero, P. (2018a). Correlated insulator behaviour at half-filling in magic-angle graphene superlattices. Nature, 556, 80–84.
Cao, Y., Fatemi, V., Fang, S., Watanabe, K., Taniguchi, T., Kaxiras, E., & Jarillo-Herrero, P. (2018b). Unconventional superconductivity in magic-angle graphene superlattices. Nature, 556, 43–50.
Crini, G. J. (2003). Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour Technol, 90, 193–198.
Cui, T., Mukherjee, S., Cao, C., Sudeep, P. M., Tam, J., Ajayan, P. M., Singh, C. V., Sun, Y., & Filleter, T. J. (2018). Effect of lattice stacking orientation and local thickness variation on the mechanical behavior of few layer graphene oxide. Carbon, 136, 168–175.
Dikin, D. A., Stankovich, S., Zimney, E. J., Piner, R. D., & Ruoff, R. S. J. (2007). Preparation and characterization of graphene oxide paper. Nature, 448, 457–460.
Dreyer, D. R., Park, S., Bielawski, C. W., & Ruoff, R. S. J. (2010). The chemistry of graphene oxide. Chem Soc Rev, 39, 228–240.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. J. (1956). Colorimetric method for determination of sugars and related substances. Anal Chem, 28, 350–356.
Dutta, S., Bohre, A., Zheng, W., Jenness, G. R., Nunez, M., Saha, B., & Vlachos, D. G. J. (2017). Solventless C-C coupling of low carbon furanics to high carbon fuel precursors using an improved graphene oxide carbocatalyst. ACS Catal, 7, 3905–3915.
Ferrari, A. J. (2007). Raman spectroscopy of graphene and graphite: disorder, electron phonon coupling, doping and nonadiabatic effects. Solid State Commun, 143, 47–57.
Ferrari, A., Meyer, J. C., Scardaci, V., Casiraghi, C., Lazzeri, M., Mauri, F., Piscanec, S., Jiang, D., Novoselov, K. S., & Roth, S. J. (2006). Raman spectrum of graphene and graphene layers. Phys Rev Lett, 97, 187401.
Gurarslan, A., Joijode, A. S., & Tonelli, A. E. J. (2012). Polymers coalesced from their cyclodextrin inclusion complexes: what can they tell us about the morphology of melt-crystallized polymers? J Polym Sci Polym Phys, 50, 813–823.
Ho, Y. S., & Mckay, G. (1998). A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ, 76, 332–340.
Houmller, J., Wanko, M., Rubio, A., & Nielsen, S. B. (2015). Effect of a single water molecule on the electronic absorption by O- and P-pitrophenolate: a shift to the red or to the blue? J Phys Chem A, 119, 11498–11503.
Hu, X., Yu, Y., Wang, Y., Zhou, J., & Song, L. J. (2015). Separating nano graphene oxide from the residual strong-acid filtrate of the modified Hummers method with alkaline solution. Appl Surf Sci, 329, 83–86.
Hummers, W. S., & Offeman, R. E. (1958). Preparation of Graphitic Oxide. J Am Chem Soc, 80, 1339.
Jiang, L. H., Liu, Y. G., Liu, S. B., Hu, X. J., Zeng, G. M., Hu, X., Liu, S. M., Liu, S. H., Huang, B. Y., & Li, M. F. (2017). Fabrication of b-cyclodextrin/poly (L-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17b-estradiol. Chem Eng J, 308, 597–605.
Kudin, K. N., Ozbas, B., Schniepp, H. C., Prudhomme, R. K., Aksay, I. A., & Car, R. J. (2008). Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett, 8, 36–41.
Langmuir, I. (1917). The constitution and fundamental properties of solids and liquids. J Am Chem Soc, 183, 102–105.
Li, J. M., Meng, X. G., Hu, C. W., & Du, J. (2009). Adsorption of phenol, p-chlorophenol and p-nitrophenol onto functional chitosan. Bioresour Technol, 100, 1168–1173.
Li, K., Li, Y., & Zheng, Z. (2010). Kinetics and mechanism studies of p-nitroaniline adsorption on activated carbon fibers prepared from cotton stalk by NH4H2PO4 activation and subsequent gasification with steam. J Hazard Mater, 178, 553–559.
Li, H., Tao, L., Huang, F., Sun, Q., Zhao, X., Han, J., Shen, Y., & Wang, M. J. (2017). Enhancing efficiency of perovskite solar cells via surface passivation with graphene oxide interlayer. ACS Appl Mater Interfaces, 9, 38967–38976.
Liu, Z., Robinson, J. T., Sun, X., & Dai, H. J. (2008). PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc, 130, 10876–10877.
Liu, Z., Xu, Y., Zhang, X., Zhang, X., Chen, Y., & Tian, J. J. (2009). Porphyrin and fullerene covalently functionalized graphene hybrid materials with large nonlinear optical properties. J Phys Chem B, 113, 9681–9686.
Liu, Y., Yu, D., Zeng, C., Miao, Z., & Dai, L. J. (2010). Biocompatible graphene oxide-based glucose biosensors. Langmuir, 26, 6158–6160.
Ma, Y. X., Shao, W. J., Sun, W., Kou, Y. L., Li, X., & Yang, H. P. (2018). One-step fabrication of β-cyclodextrin modified magnetic graphene oxide nanohybrids for adsorption of Pb(II), Cu(II) and methylene blue in aqueous solutions. Appl Surf Sci, 459, 544–553.
Maktedar, S. S., Mehetre, S. S., Avashthi, G., & Singh, M. J. (2017). In situ sonochemical reduction and direct functionalization of graphene oxide: a robust approach with thermal and biomedical applications. Ultrason Sonochem, 34, 67–77.
Mirkhani, V., Tangestaninejad, S., Yadollahi, B., & Alipanah, L. J. (2004). Efficient regio- and stereoselective ring opening of epoxides with alcohols, acetic acid and water catalyzed by ammonium decatungstocerate(IV). Cheninform, 35, 8213–8218.
Ogoshi, T., Ichihara, Y., Yamagishi, T.-A., & Nakamoto, Y. J. (2010). Supramolecular polymer networks from hybrid between graphene oxide and per-6-amino-β-cyclodextrin. Chem Commun, 46, 6087–6089.
Rahmani, F., Mahdavi, M., Nouranian, S., & Alostaz, A. J. (2017). Confinement effects on the thermal stability of poly(ethylene oxide)/graphene nanocomposites: a reactive molecular dynamics simulation study. J Polym Sci Polym Phys, 55, 1026–1035.
Samuel, M. S., Selvarajan, E., Subramaniam, K., Mathimani, T., Seethappan, S., & Pugazhendhi, A. (2020). Synthesized β-cyclodextrin modified graphene oxide (β-CD-GO) composite for adsorption of cadmium and their toxicity profile in cervical cancer (HeLa) cell lines. Process Biochem, 93, 28–35.
Stankovich, S., Piner, R. D., Nguyen, S. T., & Ruoff, R. S. J. (2006). Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon, 44, 3342–3347.
Szejtli, J. (1998). Introduction and general overview of cyclodextrin chemistry. Chem Rev, 98, 1743–1754.
Velmurugan, M., Karikalan, N., Chen, S. M., & Dai, Z. C. (2017). Studies on the influence of b-cyclodextrin on graphene oxide and its synergistic activity to the electrochemical detection of nitrobenzene. J Colloid Interface Sci, 490, 365–371.
Wang, G. H., Luo, Q. Y., Dai, J. L., & Deng, N. S. (2020). Adsorption of dichromate ions from aqueous solution onto magnetic graphene oxide modified by β-cyclodextrin. Environ Sci Pollut Res, 27, 30778–30788.
Zha, F., Li, S., & Chang, Y. J. (2008). Preparation and adsorption property of chitosan beads bearing β-cyclodextrin cross-linked by 1, 6-hexamethylene diisocyanate. Carbohydr Polym, 72, 456–461.
Zha, F., Huang, W., Wang, J., Chang, Y., Ding, J., & Ma, J. (2013). Kinetic and thermodynamic aspects of arsenate adsorption on aluminum oxide modified palygorskite nanocomposites. Chem Eng J, 215–216, 579–585.
Zheng, H. L., Gao, Y., Zhu, K. R., Wang, Q., Wakeel, M., Wahid, A., Alharbi, N. S., & Chen, C. L. (2018). Investigation of the adsorption mechanisms of Pb(II) and 1-naphthol by b-cyclodextrin modified graphene oxide nanosheets from aqueous solution. J Colloid Interface Sci, 530, 154–162.
Funding
The authors received financial support from the Young Teacher Research Group Foundation of Northwest Normal University (NWNU-LKQN-18-21) and the Nature Science Fund of China (No. 21865031).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, H., Zeng, H., Zha, F. et al. Synthesis of Graphene Oxide–Supported β-Cyclodextrin Adsorbent for Removal of p-Nitrophenol. Water Air Soil Pollut 231, 495 (2020). https://doi.org/10.1007/s11270-020-04865-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04865-8