Abstract
Purpose
To visualize SARS-CoV-2 host receptors ACE2 and CD147 on human oocytes and blastocysts.
Methods
Immunohistochemistry and confocal microscopy on human primary oocytes and pre (5 days post fertilization (dpf5) and (dpf6))- and peri (dpf7)-implantation blastocysts donated to research.
Results
SARS-CoV-2 host receptors ACE2 and CD147 are present on the membrane of trophectoderm, epiblast and hypoblast cells in human blastocysts. CD147 is also present on the oolemma.
Conclusion
Theoretically, the earliest stages of embryonic development may be vulnerable for SARS-CoV-2 infection.
Similar content being viewed by others
Introduction
Since December 2019, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causing corona virus disease 2019 (COVID-19) has spread over the globe. COVID-19 can be complicated by pneumonia, respiratory failure, systemic inflammation and coagulopathy [1]. The mortality rate is highest in elderly patients and those with comorbidities including obesity, diabetes and cardiovascular diseases. People at reproductive age usually experience mild or no symptoms. At current few data is available on the maternal and neonatal outcome after SARS-CoV-2 infection. In a small study, no adverse outcomes were reported in late third trimester pregnancies (37–41 weeks) [2]. However, a systematic review and meta-analysis on pregnancies and neonatal outcomes reported a higher risk of preterm birth, preeclampsia, caesarean section and perinatal death if the infection occurs in the early third trimester [3]. Another study reported an increased rate of maternal vascular hypoperfusion and intervillous thrombi in placentas of women with COVID-19 [4]. SARS-CoV-2 RNA [5, 6] and spike protein [6] have been found in syncytiotrophoblast of placentas from COVID-19 patients. Finally, two cases have been reported demonstrating viral RNA in the mother, neonate and placenta suggesting vertical transmission [5]. Little is known about maternal and neonatal outcomes after SARS-CoV-2 infection in the first and second trimester of pregnancy.
During the acute phase of the pandemic, the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) advised IVF centres worldwide to discontinue their activities, except for urgent cases, e.g. oncofertility. This has been decided to ensure the safety of patients and professional staff. In the meantime, IVF activities have restarted, yet vertical transmission of SARS-CoV-2 during pregnancy remains a controversial issue. It is currently unknown whether the virus might interfere with fertilization, embryo development, implantation and/or early pregnancy.
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as a receptor to enter host cells [1, 7]. ACE2 is present in many tissues such as the heart, cornea, colon and lung [8]. Recently, CD147 or Basigin (BSG) has been identified as potential additional host receptor for viral entry [9]. This highly glycosylated transmembrane protein is also present in many tissues, including the testis, uterus, ovaries and placenta. The functional role and importance of this new (co-) receptor for SARS-CoV-2 is not understood. ACE2 and CD147 transcripts have been found in human zygotes and preimplantation embryos [10], suggesting that they may have the machinery to facilitate SARS-CoV-2 entrance. The aim of our study was to visualize SARS-CoV-2 receptors ACE2 and CD147 on human oocytes and blastocysts.
Results
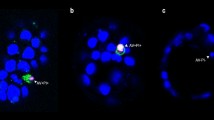
Vertical transmission implies that viral receptors are present on the interface between the mother and the embryo/foetus. In humans, early embryogenesis can only be studied in vitro on supernumerary IVF embryos that are donated to research. Here we visualize for the first time ACE2 and CD147 proteins on human oocytes and blastocysts by immunohistochemistry and confocal microscopy (Fig. 1). In primary oocytes, CD147 is mainly present on the oolemma, whereas ACE2 is absent. In pre (5 days post fertilization (dpf5) and (dpf6))- and peri (dpf7)-implantation blastocysts, CD147 and ACE2 are present on the membrane of trophectoderm and hypoblast cells, which will both contribute to the embryonic part of the placenta (chorion). Both receptors are also present on the membrane of epiblast cells, which will ultimately become the embryo proper.
Immunofluorescent staining and confocal microscopy for SARS-CoV-2 host receptors ACE2 and CD147 on human oocytes and blastocysts. (A) Co-staining for CD147 (green) and ACE2 (magenta) shows the presence of CD147 on the membrane of primary oocytes (germinal vesicle or GV) the day of oocyte retrieval (n = 13) (b, c); the ACE2 signal is absent (n = 9) (d, e). CD147 is also present on cumulus cells (CC) and on the zona pellucida (ZP) (b, c). Phase contrast image (a). Co-staining for CD147 (green), ACE2 (magenta) and SOX17 (grey) to distinguish between SOX17-positive hypoblast cells and SOX17-negative epiblast cells 5 days post fertilization (dpf5) (g–j) and dpf6 (l–o). Staining for CD147 shows that the signal is strong on the membrane of trophectoderm (TE) cells and inner cell mass (ICM) on dpf5 (n = 8) (g, i). The signal is also present on the membrane of TE, epiblast and hypoblast cells on dpf6 (n = 8) (l, n). ACE2 is present on the membrane and in the cytoplasm of TE cells and ICM cells on dpf5 (n = 9) (h, j) and on the membrane and in the cytoplasm of TE, hypoblast and epiblast cells on dpf6 (n = 6) (m, o). Phase contrast images (f, k). In TE cells of blastocysts on dpf7 (n = 8), the CD147 signal is strong on the membrane (q, s, t), whereas ACE2 is present in the cytoplasm of all TE cells and on the membrane of some TE cells (r, s, t). Phase contrast images (p). Nuclei are labelled with Hoechst (blue).(B) Mouse IgG1 and rabbit IgG controls on human oocytes (b, c) and dpf5 blastocysts (e, f). Phase contrast images (a, d). Nuclei are labelled with Hoechst (blue).
Discussion
The presence of the receptors ACE2 and CD147, especially on the membrane, implicates that SARS-CoV-2 is theoretically able to bind and infect human oocytes and pre- and peri-implantation embryos. Both embryonic and extra-embryonic lineages display these receptors, suggesting that the virus might enter the embryo at distinct moments before, during and after implantation following vertical transmission. Viral exposure might cause failure of conception, miscarriage, pregnancy complications and/or congenital malformations.
At the beginning of the viral outbreak, fertility centres worldwide discontinued their non-urgent activities to ensure the safety of patients and staff in IVF clinics. The psychological burden for IVF couples, who saw their child wish deferred, was high during the pandemic. At current, IVF centres follow rules to minimize social contact during the multiple visits at the IVF clinic. Our findings indicate that theoretically SARS-CoV-2 can be transmitted from the mother to the early developing embryo. As a consequence and to prevent embryo infection in symptomatic mothers, it could be an option to postpone IVF treatment or to cryopreserve the embryo(s) until the mother is cured. In addition one could argue to screen all IVF patients for SARS-CoV-2 infection and to postpone the IVF treatment of asymptomatic patients. Finally, our study should increase the awareness of the potential risk of SARS-CoV-2 infection on human reproduction.
Methods
Human oocytes and preimplantation embryos
Human primary oocytes and embryos were donated for research with the couples’ written informed consent. Embryos obtained after conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) were included in the study. All research embryos developed from normally fertilized oocytes (2PN).
Indirect immunohistochemistry
Human vitrified dpf3 and dpf5 embryos were warmed and cultured for 24 or 48 h in Origio Sequential Blast™ medium. Fresh human oocytes and warmed embryos were individually fixed in 4% PFA for 10 min at room temperature (RT). After fixation, samples were washed in 2% BSA/PBS and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, USA) for 20 min at RT. They were incubated in 10% FBS/PBS blocking solution for 1 h at RT and subsequently incubated with primary antibody for CD147 (10 μg/ml, Abcam, ab666), ACE2 (2 μg/ml Abcam, ab15348), SOX17 (10 μg/ml, R&D Systems, AF1924) or control antibodies (mouse IgG1 isotype control and rabbit IgG, Thermo Fisher Scientific) in 2% BSA/PBS overnight at 4 °C in the dark. Oocytes and embryos were washed in 2% BSA/PBS before incubation with secondary antibody (Alexa Fluor™ donkey-anti-mouse IgG 647, Alexa Fluor™ donkey-anti-rabbit IgG 594 and Alexa Fluor™, chicken-anti-goat IgG 488, Thermo Fisher Scientific; 10 μg/ml, 2 μg/ml and 10 μg/ml, respectively) for 2 h at RT in the dark. Samples were washed in 2% BSA/PBS before incubation with Hoechst 33342 (Molecular probes®, Life Technologies) for nuclear staining and finally mounted on coated glass slides (Poly-Prep Slides, Sigma-Aldrich). Confocal scanning microscopy with LSM800 (ZEISS, Germany) was used to obtain fluorescent images.
References
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3.
Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre descriptive study. Lancet Infect Dis. 2020;20:559–64.
Di Mascio D, Khalil A, Saccone G, Rizzo, Buca D, Liberati M et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2(2):1–9. https://doi.org/10.1016/j.ajogmf.2020.100107
Shanes E, et al. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32.
Patanè L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2:100145.
Hosier H, et al. SARS-CoV-2 infection of the placenta. Preprint at medRxiv. 2020. https://doi.org/10.1101/2020.04.30.20083907.
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARSCoV-2 by full-length human ACE2. Science. 2020;367:1444–8.
Sungnak W, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–7.
Wang K, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. Preprint at bioRxiv. 2020. https://doi.org/10.1101/2020.03.14.988345.
Stirparo GG, et al. Integrated analysis of single-cell embryo data yields a unified transcriptome signature for the human pre-implantation epiblast. Development. 2018;145:dev158501.
Acknowledgements
This work was financially supported by Wetenschappelijk Fonds Willy Gepts (WFWG) of the University Hospital UZ Brussel.
Author information
Authors and Affiliations
Contributions
Van de Velde and Essahib designed the work. Essahib collected and analysed the data and Van de Velde supervised the work. Van de Velde and Essahib wrote the manuscript. Verheyen and Tournaye critically revised the manuscript.
Corresponding author
Ethics declarations
The project was approved by the Institutional Ethical Committee (BUN 143201419672) and the Federal Committee for research on human embryos in vitro (AdV_049).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Essahib, W., Verheyen, G., Tournaye, H. et al. SARS-CoV-2 host receptors ACE2 and CD147 (BSG) are present on human oocytes and blastocysts. J Assist Reprod Genet 37, 2657–2660 (2020). https://doi.org/10.1007/s10815-020-01952-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01952-x