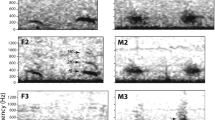
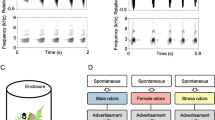
Abstract
Sexual signals in different animals are expected to be dimorphic when both sexes signal, but cases of monomorphism are known to occur, and we lack a clear understanding about the factors that modulate the level of sexual dimorphism in signals. In this study, we evaluated the hypothesis that the lack of dimorphism in sexual signals might evolve in systems experiencing temporal changing conditions of intra-sexual competition. We used the Darwin’s frog (Rhinoderma darwinii), a species with paternal care, as a model. We compared advertisement calls and examined call distinctiveness among females, pregnant and non-pregnant males in a wild population from Chiloé island, Chile. We also recorded the vocal activity of both sexes along the reproductive season. Additionally, we compared the acoustic properties of their advertisement calls in terms of sexual distinctiveness and individual repeatability. We found that the proportion of females and pregnant males vocalizing changed over time following distinct patterns. Females produced calls with lower dominant frequency and longer note and call durations than males, and these acoustic differences were related to body size differences between sexes, but only dominant frequency contributed significantly to the distinctiveness of calls between sexes. Also, individual repeatability was high, indicating that calling can be relevant for social recognition. Overall, our results suggest that mutual selective pressures could be involved in the limited dimorphism of the advertisement calls in Darwin’s frogs, as the sex ratio of individuals vocalizing (i.e. females vs. reproductive males) is reversed along the breeding period.
Significance statement
Whether sexually monomorphic signals are evidence of adaptive mutual choice or a by-product of genetic constraints on females remains as an open question. In species with exclusive parental care of males, it would be expected that males and females alternate their reproductive availability while performing slightly differentiated sexual signals. Using acoustic recordings and capture-recapture data of the Darwin’s frog, we found that advertisement calls of this frog tend to be monomorphic. Interestingly, the males performing parental care were calling actively and the population had a clear bias in the number of males. Males and females of this endangered frog called actively, but the vocalization rate of each sex peaked at different times along the breeding season. These findings open new questions about the mechanisms of sexual recognition under restricted signal dimorphism.





Similar content being viewed by others
References
Arak A (1988) Sexual size dimorphism in body size: a model and a test. Evolution 42:820–825
Atsumi K, Kishida O, Koizumi I (2019) Visual preference of males for conspecific mates in mutually ornamented fish: possible support for the species recognition hypothesis. J Ethol 37:353–362
Bang A, Gadagkar R (2012) Reproductive queue without overt conflict in the primitively eusocial wasp Ropalidia marginata. P Natl Acad Sci USA 109:14494–14499
Bee MA (2016) Social recognition in anurans. In: Bee MA, Miller CT (eds) Psychological mechanisms in animal communication. Springer, Cham, pp 169–221
Berglund A, Widemo MS, Rosenqvist G (2005) Sex-role reversal revisited: choosy females and ornamented, competitive males in a pipefish. Behav Ecol 16:649–655
Bosch J, Márquez R (1996) Acoustic competition in male midwife toads Alytes obstetricans and Alytes cisternasii: response to neighbor size and calling rate. Implications for female choice. Ethology 102:841–855
Bourke J, Barrientos C, Ortiz JC, Busse K, Böhme W, Bakker TCM (2011) Colour change in Darwin's frogs (Rhinoderma darwinii, Duméril and Bibron, 1841) (Anura: Rhinodermatidae). J Nat Hist 45:2661–2668
Burmeister S, Wilczynski W (2000) Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea). Horm Behav 38:201–209
Bush SL (1996) Why is double clutching rare in the Majorcan midwife toad? Anim Behav 52:913–922
Busse K (2003) Fortpflanzungsbiologie von Rhinoderma darwinii (Anura: Rhinodermatidae) und die stammesgeschichtliche und funktionelle Verkettung der einzelnen Verhaltensabläufe. Bonn Zool Beitr 51:3–34
Capranica RR (1965) The evoked vocal response of the bullfrog. MIT Press, Cambridge
Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assessment 6:284–290
Clutton-Brock T (2007) Sexual selection in males and females. Science 318:1882–1885
Clutton-Brock TH, Huchard E (2013) Social competition and selection in males and females. Phil Trans R Soc B 368:20130074
Colleye O, Parmentier E (2012) Overview on the diversity of sounds produced by clownfishes (Pomacentridae): importance of acoustic signals in their peculiar way of life. PLoS ONE 7:e49179
Collins S (2004) Vocal fighting and flirting: the functions of birdsong. In: Marler P, Slabbekoorn H (eds) Nature's music: the science of birdsong. Academic Press, San Diego, pp 39–79
Crawley MJ (2013) The R Book, 2nd edn. Wiley, Chichester
Crump ML (2002) Natural history of Darwin’s frog, Rhinoderma darwinii. Herpetol Nat Hist 9:21–30
Cuadrado M (2000) Body colors indicate the reproductive status of female common chameleons: experimental evidence for the intersex communication function. Ethology 106:79–91
Cui J, Wang Y, Brauth S, Tang Y (2010) A novel female call incites male-female interaction and male-male competition in the Emei music frog, Babina daunchina. Anim Behav 80:181–187
Emerson SB, Boyd SK (1999) Mating vocalizations of female frogs: control and evolutionary mechanisms. Brain Behav Evol 53:187–197
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Emlen ST, Wrege PH, Webster MS (1998) Cuckoldry as a cost of polyandry in the sex–role–reversed Wattled jacana, Jacana jacana. Proc R Soc Lond B 265:2359–2364
Ey E, Pfefferle D, Fischer J (2007) Do age-and sex-related variations reliably reflect body size in non-human primate vocalizations? A review. Primates 48:253–267
Forsgren E, Amundsen T, Borg ÅA, Bjelvenmark J (2004) Unusually dynamic sex roles in a fish. Nature 429:551–554
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks
Geberzahn N, Goymann W, Muck C, ten Cate C (2009) Females alter their song when challenged in a sex-role reversed bird species. Behav Ecol Sociobiol 64:193–204
Gheusi G, Bluthé RM, Goodall G, Dantzer R (1994) Social and individual recognition in rodents: methodological aspects and neurobiological bases. Behav Process 33:59–87
Goldberg J, Barrasso DA, Agostini MG, Quinzio S (2016) Vocal sac development and accelerated sexual maturity in the lesser swimming frog, Pseudis minuta (Anura, Hylidae). Zoology 119:489–499
Goyes Vallejos J, Grafe TU, Ahmad Sah HH, Wells KD (2017) Calling behavior of males and females of a Bornean frog with male parental care and possible sex-role reversal. Behav Ecol Sociobiol 71:95
Goymann W, Wittenzellner A, Wingfield JC (2004) Competing females and caring males. Polyandry and sex-role reversal in African black coucals, Centropus grillii. Ethology 110:807–823
Gwynne DT, Simmons LW (1990) Experimental reversal of courtship roles in an insect. Nature 346:172–174
Hare RM, Simmons LW (2020) Ecological determinants of sex roles and female sexual selection. Adv Stud Behav 52:1–28
Jimenez de la Espada DM (1872) Sobre la reproducción del Rhinoderma darwinii. An Soc Hist Nat Madrid 1:139–151
Karson M, Martell T (1980) On the interpretation of individual variables in multiple discriminant analysis. J Financ Quant Anal 15:211–217
Kasumovic MM, Bruce MJ, Andrade MC, Herberstein ME (2008) Spatial and temporal demographic variation drives within-season fluctuations in sexual selection. Evolution 62:2316–2325
Keen S, Meliza CD, Pilowsky J, Rubenstein DR (2016) Song in a social and sexual context: vocalizations signal identity and rank in both sexes of a cooperative breeder. Front Ecol Evol 4:46
Köhler J, Jansen M, Rodríguez A, Kok PJR, Toledo LF, Emmrich M, Glaw F, Haddad CFB, Rödel MO, Vences M (2017) The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa 4251:1–124
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Kokko H, Johnstone RA (2002) Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Phil Trans R Soc B 357:319–330
Kokko H, Monaghan P (2001) Predicting the direction of sexual selection. Ecol Lett 4:159–165
Kondo N, Watanabe S (2009) Contact calls: information and social function. Jpn Psychol Res 51:197–208
Kupfer A (2007) Sexual size dimorphism in amphibians: an overview. In: Fairbairn DJ, Blanckenhorn WU, Székely T (eds) Sex, size and gender roles. Oxford University Press, New York, pp 50–60
Kvarnemo C (1994) Temperature differentially affects male and female reproductive rates in the sand goby: consequences for operational sex ratio. Proc R Soc Lond B 256:151–156
Kvarnemo C, Ahnesjo I (1996) The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408
Kvarnemo C, Moore GI, Jones AG (2006) Sexually selected females in the monogamous Western Australian seahorse. Proc R Soc Lond B 274:521–525
Laidre ME, Johnstone RA (2013) Animal signals. Curr Biol 23:R829–R833
Lamba S, Chandrasekhar K, Gadagkar R (2008) Signaling hunger through aggression - the regulation of foraging in a primitively eusocial wasp. Naturwissenschaften 95:677–680
Langmore NE (1998) Functions of duet and solo songs of female birds. Trends Ecol Evol 13:136–140
Langmore NE, Bennett ATD (1999) Strategic concealment of sexual identity in an estrilid finch. Proc R Soc Lond B 266:543–550
Lea JM, Dyson M, Halliday TR (2001) Calling by male midwife toads stimulates females to continue maturing their eggs. Anim Behav 61:373–377
Lea J, Halliday TR, Dyson M (2003) The mating strategy of Alytes muletensis: some males are less ready to mate than females. Amphibia-Reptilia 24:169–180
Lemaître JF, Ronget V, Tidière M, Allainé D, Berger V, Cohas A, Colchero F, Conde DA, Garratt M, Liker A, Marais GAB, Scheuerlein A, Székely T, Gaillard JM (2020) Sex differences in adult lifespan and aging rates of mortality across wild mammals. P Natl Acad Sci USA 117:8546–8553
Lynch KS, Wilczynski W (2006) Social regulation of plasma estradiol concentration in a female anuran. Horm Behav 50:101–106
Márquez R (1990) Male parental care, sexual selection, and the mating system of the midwife toads (Alytes cisternasii and Alytes obstetricans). Dissertation, University of Chicago
Márquez R (1993) Male reproductive success in two midwife toads, Alytes obstetricans and A. cisternasii. Behav Ecol Sociobiol 32:283–291
Monnet JM, Cherry MI (2002) Sexual size dimorphism in anurans. Proc R Soc Lond B 269:2301–2307
Naes T, Mevik BH (2001) Understanding the collinearity problem in regression and discriminant analysis. J Chemometr 15:413–426
Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE (2014) Female song is widespread and ancestral in songbirds. Nat Commun 5:3379
Ord TJ, Stuart-Fox D (2006) Ornament evolution in dragon lizards: multiple gains and widespread losses reveal a complex history of evolutionary change. J Evol Biol 19:797–808
Owens IP, Thompson DB (1994) Sex differences, sex ratios and sex roles. Proc R Soc Lond B 258:93–99
Penna M, Moreno-Gómez FN (2015) Contrasting propagation of natural calls of two anuran species from the South American temperate forest. PLoS ONE 10:e0134498
Penna M, Veloso A (1990) Vocal diversity in frogs of the South American temperate forest. J Herpetol 24:23–33
Preininger D, Handschuh S, Boeckle M, Sztatecsny M, Hödl W (2016) Comparison of female and male vocalisation and larynx morphology in the size dimorphic foot-flagging frog species Staurois guttatus. Herpetol J 26:187–197
Prudic KL, Jeon C, Cao H, Monteiro A (2011) Developmental plasticity in sexual roles of butterfly species drives mutual sexual ornamentation. Science 331:73–75
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accesed 20 September 2019
Rand AS, Dudley R (1993) Frogs in helium: the anuran vocal sac is not a cavity resonator. Physiol Zool 66:793–806
Raxworthy CJ (1990) Non-random mating by size in the midwife toad Alytes obstetricans: Bigger males carry more eggs. Amphibia-Reptilia 11:247–252
Reichard M, Smith C, Bryja J (2007) Seasonal change in the opportunity for sexual selection. Mol Ecol 17:642–651
Riebel K, Odom KJ, Langmore NE, Hall ML (2019) New insights from female bird song: towards an integrated approach to studying male and female communication roles. Biol Lett 15:20190059
Roever C, Raabe N, Luebke K, Ligges U, Szepannek G, Zentgraf M, Meyer D (2020) The klaR package. Department of Statistics, University of Dortmund, Germany. https://cran.r-project.org/web/packages/klaR/klaR.pdf
Roughgarden J (2012) The social selection alternative to sexual selection. Phil Trans R Soc B 367:2294–2303
Ryan MJ (1998) Sexual selection, receiver biases, and the evolution of sex differences. Science 281:1999–2003
Schlaepfer MA, Figueroa-Sandi R (1998) Female reciprocal calling in a Costa Rican leaf-litter frog, Eleutherodactylus podiciferus. Copeia 1998:1076–1080
Serrano JM, Penna M (2018) Sexual monomorphism in the advertisement calls of a Neotropical frog. Biol J Linn Soc 123:388–401
Serrano JM, Penna M, Soto-Azat C (2020) Individual and population variation of linear and non-linear components of the advertisement call of Darwin’s frog (Rhinoderma darwinii). Bioacoustics 29:572–589
Sheehan MJ, Bergman TJ (2016) Is there an evolutionary trade-off between quality signaling and social recognition? Behav Ecol 27:2–13
Shine R (1979) Sexual selection and sexual dimorphism in the Amphibia. Copeia 1979:297–306
Soto-Azat C, Valenzuela-Sánchez A, Collen B, Rowcliffe JM, Veloso A, Cunningham AA (2013) The population decline and extinction of Darwin´s frogs. PLoS ONE 8:e66957
Stewart MM, Rand AS (1991) Vocalizations and the defence of retreat sites by male and female frogs, Eleutherodactylus coqui. Copeia 1991:1013–1024
Stoddard PK, Beecher MD, Willis MS (1988) Response of territorial male song sparrows to song types and variations. Behav Ecol Sociobiol 22:125–130
ten Hagen L, Rodríguez A, Menke N, Göcking C, Bisping M, Frommolt KH, Ziegler T, Bonkowski M, Vences M (2016) Vocalizations in juvenile anurans: common spadefoot toads (Pelobates fuscus) regularly emit calls before sexual maturity. Sci Nat 103:75
Tobias JA, Montgomerie R, Lyon BE (2012) The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil Trans R Soc B 367:2274–2293
Trail PW (1990) Why should lek-breeders be monomorphic? Evolution 44:1837–1852
Valenzuela-Sánchez A, Harding G, Cunningham AA, Chirgwin C, Soto-Azat C (2014) Home range and social analyses in a mouth brooding frog: testing the coexistence of paternal care and male territoriality. J Zool 294:215–223
Valenzuela-Sánchez A, Cayuela H, Schmidt BR, Cunningham AA, Soto-Azat C (2019) Slow natal dispersal across a homogeneous landscape suggests the use of mixed movement behaviours during dispersal in the Darwin's frog. Anim Behav 150:77–86
Venables WN, Ripley BD (2002) Modern Applied Statistics with S, 4th edn. Springer, New York
Vincent A, Ahnesjö I, Berglund A (1994) Operational sex ratios and behavioural sex differences in a pipefish population. Behav Ecol Sociobiol 34:142–435
Wells KD (2007) The ecology and behavior of amphibians. Chicago University Press, Chicago
West-Eberhard MJ (1983) Sexual selection, social competition, and speciation. Q Rev Biol 58:155–183
Wolak ME, Fairbairn DJ, Paulsen YR (2012) Guidelines for estimating repeatability. Methods Ecol Evol 3:129–137
Woolbright LL (1985) Anuran size dimorphism: reply to Sullivan. Am Nat 125:741–743
Møller A, Birkhead T (1993) Female control of paternity. Trends Ecol Evol 8:100-104
Platz JE, Forester DC (1988) Geographic variation in mating call among the four subspecies of the chorus frog: Pseudacris triseriata (Wied). Copeia 1988(4):1062-1066
Couzin ID, Krause J (2003) Self-organization and collective behavior in vertebrates. Adv Stud Behav 32:1-75
Acknowledgments
Nicolette Thompson, Mara Santoyo, María Luisa Estay, Jaime Beltrand and the Palacios family helped in obtaining data. Comments from two anonymous reviewers significantly improved the manuscript. This manuscript is dedicated to the people of Inio, Chiloé, and their unbeatable spirit (Este manuscrito está dedicado al pueblo de Inio, Chiloé, y su espíritu imbatible).
Funding
JMS was funded by Becas al extranjero Consejo Nacional de Ciencia y Tecnología (N° 312160) and Beca Nacional para Extranjeros Comisión Nacional de Investigación Científica y Tecnológica (N° 63130134). CA was funded by Fondecyt Project N° 1181758. During manuscript writing, JMS was supported by a postdoct grant at UCM, and AV-S was supported by grant FONDECYT de postdoctorado N° 3180107.
Author information
Authors and Affiliations
Contributions
JMS designed the study, collected and analysed the data and wrote manuscript drafts. MP contributed to the experimental design, data collection and manuscript writing. CA and MAM facilitated fieldwork, funding and manuscript writing. AV-S contributed to the manuscript writing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
Ethics approval
This study was carried out in accordance with the Chilean law (Servicio Agrícola y Ganadero permit N° 9822/2015). The Ethics Committee of the Faculty of Sciences of the University of Chile approved the guidelines followed to carry out this study. All applicable international, national, and/or institutional guidelines for the use of animals were followed.
Additional information
Communicated by A. Taylor Baugh
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 207 kb)
Rights and permissions
About this article
Cite this article
Serrano, J.M., Penna, M., Valenzuela-Sánchez, A. et al. Monomorphic call structure and dimorphic vocal phenology in a sex-role reversed frog. Behav Ecol Sociobiol 74, 127 (2020). https://doi.org/10.1007/s00265-020-02903-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02903-3