Abstract
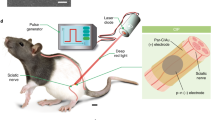
Activation of nociceptor sensory neurons by noxious stimuli both triggers pain and increases capillary permeability and blood flow to produce neurogenic inflammation1,2, but whether nociceptors also interact with the immune system remains poorly understood. Here we report a neurotechnology for selective epineural optogenetic neuromodulation of nociceptors and demonstrate that nociceptor activation drives both protective pain behavior and inflammation. The wireless optoelectronic system consists of sub-millimeter-scale light-emitting diodes embedded in a soft, circumneural sciatic nerve implant, powered and driven by a miniaturized head-mounted control unit. Photostimulation of axons in freely moving mice that express channelrhodopsin only in nociceptors resulted in behaviors characteristic of pain, reflecting orthodromic input to the spinal cord. It also led to immune reactions in the skin in the absence of inflammation and potentiation of established inflammation, a consequence of the antidromic activation of nociceptor peripheral terminals. These results reveal a link between nociceptors and immune cells, which might have implications for the treatment of inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw data that support the findings of this study will be made available upon reasonable request to the corresponding authors. Source data are provided with this paper.
References
Talbot, S., Foster, S. L. & Woolf, C. J. Neuroimmunity: physiology and pathology. Annu. Rev. Immunol. 34, 421–447 (2016).
Chiu, I. M., von Hehn, C. A. & Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067 (2012).
Sharma, N. et al. The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398 (2020).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Xu, X., Mee, T. & Jia, X. New era of optogenetics: from the central to peripheral nervous system. Crit. Rev. Biochem. Mol. Biol. 55, 1–16 (2020).
Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Zhang, Y. et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv. 5, eaaw5296 (2019).
Mickle, A. D. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Keppeler, D. et al. Multichannel optogenetic stimulation of the auditory pathway using microfabricated LED cochlear implants in rodents. Sci. Transl. Med. 12, eabb8086 (2020).
Burton, A. et al. Wireless, battery-free subdermally implantable photometry systems for chronic recording of neural dynamics. Proc. Natl Acad. Sci. USA 117, 2835–2845 (2020).
Lu, L. et al. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proc. Natl Acad. Sci. USA 115, E1374–E1383 (2018).
Kim, T.-I. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Pinho-Ribeiro, F. A., Verri, W. A. & Chiu, I. M. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol. 38, 5–19 (2017).
Ronchetti, S., Migliorati, G. & Delfino, D. V. Association of inflammatory mediators with pain perception. Biomed. Pharmacother. 96, 1445–1452 (2017).
Ghasemlou, N., Chiu, I. M., Julien, J.-P. & Woolf, C. J. CD11b+Ly6G− myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc. Natl Acad. Sci. USA 112, E6808–E6817 (2015).
Marino, M. J. et al. Botulinum toxin B in the sensory afferent: transmitter release, spinal activation, and pain behavior. Pain 155, 674–684 (2014).
Lewin, G. R., Lisney, S. J. W. & Mendell, L. M. Neonatal anti-NGF treatment reduces the Aδ- and C-fibre evoked vasodilator responses in rat skin: evidence that nociceptor afferents mediate antidromic vasodilatation. Eur. J. Neurosci. 4, 1213–1218 (1992).
Cohen, J. A. et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–932 (2019).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 (2017).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Patil, M. J., Hovhannisyan, A. H. & Akopian, A. N. Characteristics of sensory neuronal groups in CGRP-cre-ER reporter mice: comparison to Nav1.8-cre, TRPV1-cre and TRPV1-GFP mouse lines. PLoS ONE 13, e0198601 (2018).
Makadia, P. A. et al. Optogenetic activation of colon epithelium of the mouse produces high-frequency bursting in extrinsic colon afferents and engages visceromotor responses. J. Neurosci. 38, 5788–5798 (2018).
Cavanaugh, D. J. et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 31, 5067–5077 (2011).
Storozhuk, M. V., Moroz, O. F. & Zholos, A. V. Multifunctional TRPV1 ion channels in physiology and pathology with focus on the brain, vasculature, and some visceral systems. Biomed. Res. Int. 2019, 1–12 (2019).
Schonle, P., Fateh, S., Burger, T. & Huang, Q. A power-efficient multi-channel PPG ASIC with 112dB receiver DR for pulse oximetry and NIRS. In Proc. 2017 IEEE Custom Integrated Circuits Conference (CICC) 1–4 (Institute of Electrical and Electronics Engineers, 2017).
Nikolic, K. et al. Photocycles of channelrhodopsin-2. Photochem. Photobiol. 85, 400–411 (2009).
Michoud, F. et al. Optical cuff for optogenetic control of the peripheral nervous system. J. Neural Eng. 15, 015002 (2018).
Browne, L. E. et al. Time-resolved fast mammalian behavior reveals the complexity of protective pain responses. Cell Rep. 20, 89–98 (2017).
Daou, I. et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J. Neurosci. 33, 18631–18640 (2013).
Cornett, P. M., Matta, J. A. & Ahern, G. P. General anesthetics sensitize the capsaicin receptor transient receptor potential V1. Mol. Pharmacol. 74, 1261–1268 (2008).
Prabhakar, A., Vujovic, D., Cui, L., Olson, W. & Luo, W. Leaky expression of channelrhodopsin-2 (ChR2) in Ai32 mouse lines. PLoS ONE 14, e0213326 (2019).
Cavanaugh, D. J. et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl Acad. Sci. USA 106, 9075–9080 (2009).
Montgomery, K. L., Iyer, S. M., Christensen, A. J., Deisseroth, K. & Delp, S. L. Beyond the brain: optogenetic control in the spinal cord and peripheral nervous system. Sci. Transl. Med. 8, 337rv5 (2016).
Huang, X. et al. Materials strategies and device architectures of emerging power supply devices for implantable bioelectronics. Small 16, 1902827 (2020).
Zheng, H. et al. A shape-memory and spiral light-emitting device for precise multisite stimulation of nerve bundles. Nat. Commun. 10, 2790 (2019).
Maimon, B. E., Sparks, K., Srinivasan, S., Zorzos, A. N. & Herr, H. M. Spectrally distinct channelrhodopsins for two-colour optogenetic peripheral nerve stimulation. Nat. Biomed. Eng. 2, 485–496 (2018).
Schonle, P. et al. A multi-sensor and parallel processing SoC for miniaturized medical instrumentation. IEEE J. Solid-State Circuits 53, 2076–2087 (2018).
Mcintosh, R. L. & Anderson, V. A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys. Rev. Lett. 05, 129–151 (2010).
Dong, N. et al. Opto-electro-thermal optimization of photonic probes for optogenetic neural stimulation. J. Biophotonics 11, e201700358 (2018).
Acknowledgements
The authors thank N. Andrews and L. Barrett for technical assistance, G. Courtine and his team (G-Lab, EPFL) for their advice on the surgical procedure and M. Stoeckel, A. Guillet and V. Ruhaut (Neuronal Microsystems Platform, Wyss Center) for help and advice on microfabrication. Further thanks go to T. Kleier for PCB assembly and device measurement support and M. Zahner for his help with antenna characterization. For funding, we would like to acknowledge a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (109372/Z/15/Z, to L.E.B.), the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement (754354, to O.A.), the Bertarelli Foundation (to S.P.L. and C.J.W.), the Swiss National Science Foundation (BSCGI0_1578000, to S.P.L.) and the National Institutes of Health (R35NS105076, to C.J.W.).
Author information
Authors and Affiliations
Contributions
F.M., C.S., P.S., Q.H., C.J.W. and S.P.L. contributed equally to this work. F.M., C.S., S.T., S.P.L. and C.J.W. designed the study and experiments. F.M. developed, fabricated and implanted the optoelectronic devices. C.S., F.M., D.T. and A.J. designed, performed and analyzed data from the neuroimmune experiments. P.S., N.B., P.M. and Q.H. developed the wireless LED-driving unit: P.S. designed the ASIC; N.B. and P.S. designed the PCB, manufactured the devices and performed the measurements; and the Android application and the BLE SoC were programmed by P.M. and N.B., respectively. F.M. and I.F. developed the mechanical and optical characterization setups. O.A. developed the thermal FEM. R.M., M.T. and B.D. assisted during surgery and collected and analyzed behavioral data. K.G. performed histology for biocompatibility. L.E.B. established the transgenic mouse line and advised on the control of the acquisition system. F.M., C.S., P.S., Q.H., C.J.W. and S.P.L. wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Wireless micro-LED-driving unit preparation.
a, Fully assembled PCB including additional ‘wings’ for access to various signals during device firmware development and for the initial flashing of the program code onto the device. After initial flashing and thorough testing, the ‘wings’ are cut and antenna and battery are attached and soldered. b, The final device measures 8.0 x 11.0 x 5.3 mm3 and weighs 0.85 g – 1.1g after encapsulation in silicone. c, The charging station is used for turning on/off the units – a design choice by which the wireless units can be fully encapsulated and be without any mechanical components (except the connector). Multiple stations can be interconnected to each other such that a single USB port can be used for the charging of many micro-LED driving units. d, Android application screen in experiment mode and overview on the configuration ranges. Each issued current pulse is monitored, that is, the wireless unit checks if the set current is actually reached and transmits this feedback to the tablet/smartphone. e, Android application screen in maximum-current test mode and overview on the configuration ranges. This operation mode enables the user to quickly determine the condition of a micro-LED implant: A sequence of short current pulses (too short to evoke an optogenetic stimulation) is emitted to determine the maximum current that can be achieved.
Extended Data Fig. 2 Antenna design and characterization.
a, Custom antenna layout with dimensions. The antenna is encapsulated within two layers of silicone prior to PCB soldering. b, Device dummy for return loss (S11) measurements. c, Measurement setup in the anechoic chamber. The device is mounted on a turntable. d, Radiation pattern characterization with a battery-operated device dummy constantly emitting a known-power carrier. Drawn lines represent measurements at center frequency (2440 MHz) while the shades cover the full BLE bandwidth (further measurements at 2402, 2430 and 2480 MHz).
Extended Data Fig. 3 Wireless micro-LED-driving unit performances.
a, Measurement of micro-LED array current during photostimulation pulse train (2 Hz, 10 mA, 10 ms pulse duration) as typically used in the in vivo experiments. b, Micro-LED current pulses emitted during a linear, ultra-low duty-cycle (100 ppm) maximum current search run. Short (20-100 µs) pulses at a low frequency (2 Hz) are used to avoid ChR2 activation during the test. The system delivers up to a 35 mA maximum current to the micro-LEDs. c, Measured overall power consumption of the wireless LED driving unit in various operation states. d, Battery lifetime measurement for a device in advertising state that is the device is turned on, however not connected to any tablet or smartphone and does not drive any micro-LED arrays. BLE connection will reduce lifetime by ca. 10%.
Extended Data Fig. 4 Microfabrication of the flexible micro-LED array to target activation of ChR2 in vivo.
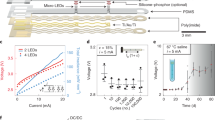
a–c, Schematic illustration of the microfabrication process. Patterning of a Ti/Au film on a polyimide substrate (a). Polyimide superstrate covering of the film followed by patterning of the polyimide stack (b). Encapsulation in PDMS and subsequent patterning of the silicone layer (c). d, Schematic cross-sections of the interconnects and micro-LED site of integration. e–g, Illustrations of the micro-LED integration process. Printing of soldering paste on the interconnect pads followed by precise placement of the micro-LED (e). The reflow of the paste ensures mechanical and electrical interfacing. Printing of polyisobuthylene (PIB) on the micro-LED surface (f). PDMS encapsulation and release from the silicon carrier (g). h–j, Representative optical micrographs, such as solder paste printing (h), micro-LED deposition (i) and activation (j). Scale bar: 250 µm. k, Relative intensity associated with micro-LED emission spectrum, or spectral flux, and ChR2 normalized response spectrum. l, Relative changes in voltage for devices placed in 67 °C saline depending on micro-LED encapsulation material. Arrays only encapsulated with PDMS fail after day 7. n = 3 devices per group; mean ± s.d.
Extended Data Fig. 5 Illustration of surgical procedures for implanting the micro-LED array.
a, Under anesthesia and using aseptic technique, a craniotomy is performed and three micro-screws are fixed on the skull. b, Following a skin incision at the thigh level, implant wires are threaded subcutaneously. Suture threads are adjusted on the implant anchoring points. c, The micro-LED implant is placed transversally to the sciatic nerve and secured in position with the suture threads. d, Muscles are closed with sutures and the implant subcutaneous connector is secured at the vicinity. e, The implant is tested intraoperatively with a short activation of the micro-LEDs. f, Photograph of 3 mice carrying a wireless optoelectronic system.
Extended Data Fig. 6 Simulation of optoelectronic-induced temperature change in silico.
a, Elements and their relative 3D geometry used to model the appropriate heat transfer. The micro-LED array is placed epineurally, distributing equally 4 micro-LEDs or heat sources on the sciatic nerve. b, Thermodynamic parameters of the elements used in the thermal model. c, Temperature changes predicted by the 3D model, presented in cross and longitudinal sections. For visualization, the simulated photostimulation parameters (100 mW/mm2, 10% activation duty cycle) exceed the ones used in the in vivo experiments. d, Distribution of optoelectronic-induced temperature change across the different elements as a function of photostimulation irradiance. e, Maximum temperature increment at the interface between inner nerve and epineurium as a function of irradiance and micro-LED activation duty cycle.
Extended Data Fig. 7 Effects of micro-LED array implantation or sham surgery on animal behaviour.
a, Elements and their relative 3D geometry used to model the appropriate heat transfer. The micro-LED array is placed epineurally, distributing equally 4 micro-LEDs or heat sources on the sciatic nerve. b, Thermodynamic parameters of the elements used in the thermal model. c, Temperature changes predicted by the 3D model, presented in cross and longitudinal sections. For visualization, the simulated photostimulation parameters (100 mW/mm2, 10% activation duty cycle) exceed the ones used in the in vivo experiments. d, Distribution of optoelectronic-induced temperature change across the different elements as a function of photostimulation irradiance. e, Maximum temperature increment at the interface between inner nerve and epineurium as a function of irradiance and micro-LED activation duty cycle.
Extended Data Fig. 8 Flow cytometric data extracted from the ipsilateral hindpaw skin with respect to the micro-LED array location.
Pseudo-colorized dot plots showing myeloid (CD45+Thy1.2-) and lymphoid (CD45+Thy1.2+) cell populations depending on experimental protocol (± CFA and ± Stim). Population frequencies of the cells in the boxed regions are shown.
Extended Data Fig. 9 Optogenetic stimulation of TRPV1-Cre–/–::ChR2+/+ mice does not result in cell mobilization in innervated hind paw skin.
Major immune cell population numbers as assessed by flow cytometry. n = 5 animals per group; two-sided unpaired t-test. Symbols represent individual mice analyzed independently. The box plots display the median and interquartile range, ‘+’ denotes the mean, and the extending whiskers, the largest and lowest observations.
Extended Data Fig. 10 Injection of CFA in nociceptor ablated animal paw skin does not result in changes in immune cell numbers.
TRPV1-Cre+/+::DTA+/+ mice were injected with 3μl CFA in left hind paw, and immune cell population numbers were assessed by flow cytometry 3 days later. n = 3 animals per group; two-sided unpaired t-test. All data represented as mean ± s.e.m.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Source data
Source Data Fig. 2
Experimental data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 2
Experimental data.
Source Data Extended Data Fig. 3
Experimental data.
Source Data Extended Data Fig. 4
Experimental data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
FEM data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Michoud, F., Seehus, C., Schönle, P. et al. Epineural optogenetic activation of nociceptors initiates and amplifies inflammation. Nat Biotechnol 39, 179–185 (2021). https://doi.org/10.1038/s41587-020-0673-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-020-0673-2
This article is cited by
-
Body-conformable light-emitting materials and devices
Nature Photonics (2024)
-
A site-selective integration strategy for microdevices on conformable substrates
Nature Electronics (2024)
-
Chronic pain can be treated — so why are millions still suffering?
Nature (2023)
-
Sensory nerves directly promote osteoclastogenesis by secreting peptidyl-prolyl cis-trans isomerase D (Cyp40)
Bone Research (2023)
-
Fatigue-resistant hydrogel optical fibers enable peripheral nerve optogenetics during locomotion
Nature Methods (2023)